Related Research Articles

In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle is emitted from an atomic nucleus, transforming the original nuclide to an isobar. For example, beta decay of a neutron transforms it into a proton by the emission of an electron accompanied by an antineutrino; or, conversely a proton is converted into a neutron by the emission of a positron with a neutrino in so-called positron emission. Neither the beta particle nor its associated (anti-)neutrino exist within the nucleus prior to beta decay, but are created in the decay process. By this process, unstable atoms obtain a more stable ratio of protons to neutrons. The probability of a nuclide decaying due to beta and other forms of decay is determined by its nuclear binding energy. The binding energies of all existing nuclides form what is called the nuclear band or valley of stability. For either electron or positron emission to be energetically possible, the energy release or Q value must be positive.

Deuterium is one of two stable isotopes of hydrogen. The nucleus of a deuterium atom, called a deuteron, contains one proton and one neutron, whereas the far more common protium has no neutrons in the nucleus. Deuterium has a natural abundance in Earth's oceans of about one atom in 6420 of hydrogen. Thus deuterium accounts for approximately 0.02% of all the naturally occurring hydrogen in the oceans, while protium accounts for more than 99.98%. The abundance of deuterium changes slightly from one kind of natural water to another.

The neutron is a subatomic particle, symbol
n
or
n0
, with no net electric charge and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons behave similarly within the nucleus, and each has a mass of approximately one atomic mass unit, they are both referred to as nucleons. Their properties and interactions are described by nuclear physics.

Nuclear fusion is a reaction in which two or more atomic nuclei are combined to form one or more different atomic nuclei and subatomic particles. The difference in mass between the reactants and products is manifested as either the release or absorption of energy. This difference in mass arises due to the difference in atomic "binding energy" between the atomic nuclei before and after the reaction. Fusion is the process that powers active or "main sequence" stars, or other high magnitude stars.
Muon-catalyzed fusion (μCF) is a process allowing nuclear fusion to take place at temperatures significantly lower than the temperatures required for thermonuclear fusion, even at room temperature or lower. It is one of the few known ways of catalyzing nuclear fusion reactions.
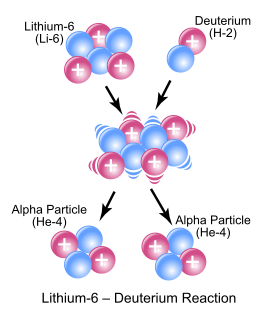
In nuclear physics and nuclear chemistry, a nuclear reaction is semantically considered to be the process in which two nuclei, or else a nucleus of an atom and a subatomic particle from outside the atom, collide to produce one or more nuclides that are different from the nuclide(s) that began the process. Thus, a nuclear reaction must cause a transformation of at least one nuclide to another. If a nucleus interacts with another nucleus or particle and they then separate without changing the nature of any nuclide, the process is simply referred to as a type of nuclear scattering, rather than a nuclear reaction.

In atomic physics, hyperfine structure is defined by small shifts and splittings in the energy levels of atoms, molecules, and ions, due to interaction between the state of the nucleus and the state of the electron clouds.
The Oppenheimer–Phillips process or strip reaction is a type of deuteron-induced nuclear reaction. In this process the neutron half of an energetic deuteron fuses with a target nucleus, transmuting the target to a heavier isotope while ejecting a proton. An example is the nuclear transmutation of carbon-12 to carbon-13.

Electron neutrinos are produced in the Sun as a product of nuclear fusion. Solar neutrinos constitute by far the largest flux of neutrinos from natural sources observed on Earth, as compared with e.g. atmospheric neutrinos or the diffuse supernova neutrino background.

The nuclear force is a force that acts between the protons and neutrons of atoms. Neutrons and protons, both nucleons, are affected by the nuclear force almost identically. Since protons have charge +1 e, they experience an electric force that tends to push them apart, but at short range the attractive nuclear force is strong enough to overcome the electromagnetic force. The nuclear force binds nucleons into atomic nuclei.
In physics, induced gamma emission (IGE) refers to the process of fluorescent emission of gamma rays from excited nuclei, usually involving a specific nuclear isomer. It is analogous to conventional fluorescence, which is defined as the emission of a photon by an excited electron in an atom or molecule. In the case of IGE, nuclear isomers can store significant amounts of excitation energy for times long enough for them to serve as nuclear fluorescent materials. There are over 800 known nuclear isomers but almost all are too intrinsically radioactive to be considered for applications. As of 2006 there were two proposed nuclear isomers that appeared to be physically capable of IGE fluorescence in safe arrangements: tantalum-180m and hafnium-178m2.
Nuclear reaction analysis (NRA) is a nuclear method of nuclear spectroscopy in materials science to obtain concentration vs. depth distributions for certain target chemical elements in a solid thin film.

The International Fusion Materials Irradiation Facility, also known as IFMIF, is a projected material testing facility in which candidate materials for the use in an energy producing fusion reactor can be fully qualified. IFMIF will be an accelerator-driven neutron source producing a high intensity fast neutron flux with a spectrum similar to that expected at the first wall of a fusion reactor using a deuterium-lithium nuclear reaction. The IFMIF project was started in 1994 as an international scientific research program, carried out by Japan, the European Union, the United States, and Russia, and managed by the International Energy Agency. Since 2007, it has been pursued by Japan and the European Union under the Broader Approach Agreement in the field of fusion energy research, through the IFMIF/EVEDA project, which conducts engineering validation and engineering design activities for IFMIF. The construction of IFMIF is recommended in the European Roadmap for Research Infrastructures Report, which was published by the European Strategy Forum on Research Infrastructures (ESFRI).
The Lambda baryons are a family of subatomic hadron particles containing one up quark, one down quark, and a third quark from a higher flavour generation, in a combination where the quantum wave function changes sign upon the flavour of any two quarks being swapped. They are thus baryons, with total isospin of 0, and have either neutral electric charge or the elementary charge +1.
Stuart Thomas Butler was an Australian nuclear physicist who served as Director of the Australian Atomic Energy Commission from 1977 until 1982, and was noted for his contributions to theoretical physics including stripping reactions, energy loss of particles in plasma and atmospheric tides induced by absorption of solar radiation in the ozone layer.

Fermilab E-906/SeaQuest is a particle physics experiment which will use Drell–Yan process to measure the contributions of antiquarks to the structure of the proton or neutron and how this structure is modified when the proton or neutron is included within an atomic nucleus.
The rms charge radius is a measure of the size of an atomic nucleus, particularly the proton distribution. It can be measured by the scattering of electrons by the nucleus. Relative changes in the mean squared nuclear charge distribution can be precisely measured with atomic spectroscopy.
The light front quantization of quantum field theories provides a useful alternative to ordinary equal-time quantization. In particular, it can lead to a relativistic description of bound systems in terms of quantum-mechanical wave functions. The quantization is based on the choice of light-front coordinates, where plays the role of time and the corresponding spatial coordinate is . Here, is the ordinary time, is one Cartesian coordinate, and is the speed of light. The other two Cartesian coordinates, and , are untouched and often called transverse or perpendicular, denoted by symbols of the type . The choice of the frame of reference where the time and -axis are defined can be left unspecified in an exactly soluble relativistic theory, but in practical calculations some choices may be more suitable than others. The basic formalism is discussed elsewhere.

James H. Coon physicist at Los Alamos Scientific Laboratory, made significant contributions to the science and study of neutron interactions. He worked on the Vera satellite project.

The perturbed γ-γ angular correlation, PAC for short or PAC-Spectroscopy, is a method of nuclear solid-state physics with which magnetic and electric fields in crystal structures can be measured. In doing so, electrical field gradients and the Larmor frequency in magnetic fields as well as dynamic effects are determined. With this very sensitive method, which requires only about 10-1000 billion atoms of a radioactive isotope per measurement, material properties in the local structure, phase transitions, magnetism and diffusion can be investigated. The PAC method is related to nuclear magnetic resonance and the Mössbauer effect, but shows no signal attenuation at very high temperatures. Today only the time-differential perturbed angular correlation (TDPAC) is used.
References
- ↑ Butler, S. T. (1950-12-15). "On Angular Distributions from (d,p) and(d,n) Nuclear Reactions". Physical Review. American Physical Society (APS). 80 (6): 1095–1096. doi:10.1103/physrev.80.1095.2. ISSN 0031-899X.
- ↑ Butler, S. T. (1951-09-24). "Angular distributions from (d,p) and (d,n) nuclear reactions". Proceedings of the Royal Society of London. Series A. Mathematical and Physical Sciences. The Royal Society. 208 (1095): 559–579. doi:10.1098/rspa.1951.0182. ISSN 0080-4630.