Related Research Articles

Gypsum is a soft sulfate mineral composed of calcium sulfate dihydrate, with the chemical formula CaSO4·2H2O. It is widely mined and is used as a fertilizer and as the main constituent in many forms of plaster, drywall and blackboard or sidewalk chalk. Alabaster, a fine-grained white or lightly tinted variety of gypsum, has been used for sculpture by many cultures including Ancient Egypt, Mesopotamia, Ancient Rome, the Byzantine Empire, and the Nottingham alabasters of Medieval England. Gypsum also crystallizes as translucent crystals of selenite. It forms as an evaporite mineral and as a hydration product of anhydrite.

Sulfur (also spelled sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula S8. Elemental sulfur is a bright yellow, crystalline solid at room temperature.

Sulfuric acid or sulphuric acid, known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, and hydrogen, with the molecular formula H2SO4. It is a colorless, odorless, and viscous liquid that is miscible with water.

The mineral pyrite, or iron pyrite, also known as fool's gold, is an iron sulfide with the chemical formula FeS2 (iron (II) disulfide). Pyrite is the most abundant sulfide mineral.

An alum is a type of chemical compound, usually a hydrated double sulfate salt of aluminium with the general formula XAl(SO
4)
2·12 H
2O, where X is a monovalent cation such as potassium or ammonium. By itself, "alum" often refers to potassium alum, with the formula KAl(SO
4)
2·12 H
2O. Other alums are named after the monovalent ion, such as sodium alum and ammonium alum.
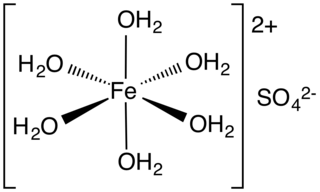
Iron(II) sulfate (British English: iron(II) sulphate) or ferrous sulfate denotes a range of salts with the formula Fe SO4·xH2O. These compounds exist most commonly as the heptahydrate (x = 7) but several values for x are known. The hydrated form is used medically to treat iron deficiency, and also for industrial applications. Known since ancient times as copperas and as green vitriol (vitriol is an archaic name for sulfate), the blue-green heptahydrate (hydrate with 7 molecules of water) is the most common form of this material. All the iron(II) sulfates dissolve in water to give the same aquo complex [Fe(H2O)6]2+, which has octahedral molecular geometry and is paramagnetic. The name copperas dates from times when the copper(II) sulfate was known as blue copperas, and perhaps in analogy, iron(II) and zinc sulfate were known respectively as green and white copperas.
Dry distillation is the heating of solid materials to produce gaseous products. The method may involve pyrolysis or thermolysis, or it may not.

Calcium sulfate (or calcium sulphate) is the inorganic compound with the formula CaSO4 and related hydrates. In the form of γ-anhydrite (the anhydrous form), it is used as a desiccant. One particular hydrate is better known as plaster of Paris, and another occurs naturally as the mineral gypsum. It has many uses in industry. All forms are white solids that are poorly soluble in water. Calcium sulfate causes permanent hardness in water.

Melanterite is a mineral form of hydrous iron(II) sulfate: FeSO4·7H2O. It is the iron analogue of the copper sulfate chalcanthite. It alters to siderotil by loss of water. It is a secondary sulfate mineral which forms from the oxidation of primary sulfide minerals such as pyrite and marcasite in the near-surface environment. It often occurs as a post mine encrustation on old underground mine surfaces. It also occurs in coal and lignite seams exposed to humid air and as a rare sublimate phase around volcanic fumaroles. Associated minerals include pisanite, chalcanthite, epsomite, pickeringite, halotrichite and other sulfate minerals.

The sulfur cycle is a biogeochemical cycle in which the sulfur moves between rocks, waterways and living systems. It is important in geology as it affects many minerals and in life because sulfur is an essential element (CHNOPS), being a constituent of many proteins and cofactors, and sulfur compounds can be used as oxidants or reductants in microbial respiration. The global sulfur cycle involves the transformations of sulfur species through different oxidation states, which play an important role in both geological and biological processes. Steps of the sulfur cycle are:

Cement kilns are used for the pyroprocessing stage of manufacture of portland and other types of hydraulic cement, in which calcium carbonate reacts with silica-bearing minerals to form a mixture of calcium silicates. Over a billion tonnes of cement are made per year, and cement kilns are the heart of this production process: their capacity usually defines the capacity of the cement plant. As the main energy-consuming and greenhouse-gas–emitting stage of cement manufacture, improvement of kiln efficiency has been the central concern of cement manufacturing technology. Emissions from cement kilns are a major source of greenhouse gas emissions, accounting for around 2.5% of non-natural carbon emissions worldwide.

Ammonium bisulfate, also known as ammonium hydrogen sulfate, is a white, crystalline solid with the formula (NH4)HSO4. This salt is the product of the half-neutralization of sulfuric acid by ammonia.

Iron(III) sulfate (or ferric sulfate), is a family of inorganic compounds with the formula Fe2(SO4)3(H2O)n. A variety of hydrates are known, including the most commonly encountered form of "ferric sulfate". Solutions are used in dyeing as a mordant, and as a coagulant for industrial wastes. Solutions of ferric sulfate are also used in the processing of aluminum and steel.

Calcium sulfite, or calcium sulphite, is a chemical compound, the calcium salt of sulfite with the formula CaSO3·x(H2O). Two crystalline forms are known, the hemihydrate and the tetrahydrate, respectively CaSO3·½(H2O) and CaSO3·4(H2O). All forms are white solids. It is most notable as the product of flue-gas desulfurization.

Millosevichite is a rare sulfate mineral with the chemical formula Al2(SO4)3. Aluminium is often substituted by iron. It forms finely crystalline and often porous masses.

Godovikovite is a rare sulfate mineral with the chemical formula: (NH4)Al(SO4)2. Aluminium can partially be substituted by iron. Hydration of godovikovite gives the ammonium alum, tschermigite. The mineral forms cryptocrystalline, often porous, masses, usually of white colour. Single crystals are very small hexagonal blades. Typical environment for godovikovite are burning coal sites (mainly dumps). There the mineral acts, together with millosevichite, as one of the main components of so-called sulfate crust.
Xitieshanite is a hydrous iron sulfate–chloride mineral with chemical formula: Fe3+(SO4)Cl·6(H2O).

2-Octanol is an organic compound with the chemical formula CH3CH(OH)(CH2)5CH3. It is a colorless oily liquid that is poorly soluble in water but soluble in most organic solvents. 2-Octanol is classified fatty alcohol. A secondary alcohol, it is chiral.
A sulfite sulfate is a chemical compound that contains both sulfite and sulfate anions [SO3]2− [SO4]2−. These compounds were discovered in the 1980s as calcium and rare earth element salts. Minerals in this class were later discovered. Minerals may have sulfite as an essential component, or have it substituted for another anion as in alloriite. The related ions [O3SOSO2]2− and [(O2SO)2SO2]2− may be produced in a reaction between sulfur dioxide and sulfate and exist in the solid form as tetramethyl ammonium salts. They have a significant partial pressure of sulfur dioxide.

Fumarole minerals are minerals which are deposited by fumarole exhalations. They form when gases and compounds desublimate or precipitate out of condensates, forming mineral deposits. They are mostly associated with volcanoes following deposition from volcanic gas during an eruption or discharge from a volcanic vent or fumarole, but have been encountered on burning coal deposits as well. They can be black or multicoloured and are often unstable upon exposure to the atmosphere.
References
- ↑ Srebrodolskiy B. I. 1989: Tainy Sezonnykh Mineralov. Nauka, Moscow
- ↑ Jambor J. L. and Grew E. S. 1991: New mineral names. American Mineralogist, 76, pp. 299-305
- ↑ Sokol E. V., Maksimova N. V., Nigmatulina E. N., Sharygin V. V. and Kalugin V. M. 2005: Combustion metamorphism. Publishing House of the SB RAS, Novosibirsk