Related Research Articles

Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference and identifiable chemical change. These reactions involve electrons moving via an electronically-conducting phase between electrodes separated by an ionically conducting and electronically insulating electrolyte.

An electrochemical cell is a device that generates electrical energy from chemical reactions. Electrical energy can also be applied to these cells to cause chemical reactions to occur. Electrochemical cells that generate an electric current are called voltaic or galvanic cells and those that generate chemical reactions, via electrolysis for example, are called electrolytic cells.

In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from naturally occurring sources such as ores using an electrolytic cell. The voltage that is needed for electrolysis to occur is called the decomposition potential. The word "lysis" means to separate or break, so in terms, electrolysis would mean "breakdown via electricity."
The term chloride refers to a compound or molecule that contains either a chlorine ion, which is a negatively charged chlorine atom, or a non-charged chlorine atom covalently bonded to the rest of the molecule by a single bond. Many inorganic chlorides are salts. Many organic compounds are chlorides. The pronunciation of the word "chloride" is.

Redox is a type of chemical reaction in which the oxidation states of a reactant change and that reduction and oxidation occur at the same time in a reaction. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state.
In chemistry, a reducing agent is a chemical species that "donates" an electron to an electron recipient.

An oxidizing agent is a substance in a redox chemical reaction that gains or "accepts"/"receives" an electron from a reducing agent. In other words, an oxidizer is any substance that oxidizes another substance. The oxidation state, which describes the degree of loss of electrons, of the oxidizer decreases while that of the reductant increases; this is expressed by saying that oxidizers "undergo reduction" and "are reduced" while reducers "undergo oxidation" and "are oxidized". Common oxidizing agents are oxygen, hydrogen peroxide, and the halogens.

A galvanic cell or voltaic cell, named after the scientists Luigi Galvani and Alessandro Volta, respectively, is an electrochemical cell in which an electric current is generated from spontaneous oxidation–reduction reactions. A common apparatus generally consists of two different metals, each immersed in separate beakers containing their respective metal ions in solution that are connected by a salt bridge or separated by a porous membrane.

An electrolytic cell is an electrochemical cell that utilizes an external source of electrical energy to force a chemical reaction that would otherwise not occur. The external energy source is a voltage applied between the cell's two electrodes; an anode and a cathode, which are immersed in an electrolyte solution. This is in contrast to a galvanic cell, which itself is a source of electrical energy and the foundation of a battery. The net reaction taking place in a galvanic cell is a spontaneous reaction, i.e., the Gibbs free energy remains -ve, while the net reaction taking place in an electrolytic cell is the reverse of this spontaneous reaction, i.e., the Gibbs free energy is +ve.
An oxyanion, or oxoanion, is an ion with the generic formula A
xOz−
y. Oxyanions are formed by a large majority of the chemical elements. The formulae of simple oxyanions are determined by the octet rule. The corresponding oxyacid of an oxyanion is the compound H
zA
xO
y. The structures of condensed oxyanions can be rationalized in terms of AOn polyhedral units with sharing of corners or edges between polyhedra. The oxyanions adenosine monophosphate (AMP), adenosine diphosphate (ADP) and adenosine triphosphate (ATP) are important in biology.

Chlorate is the common name of the ClO−
3 anion, whose chlorine atom is in the +5 oxidation state. The term can also refer to chemical compounds containing this anion, with chlorates being the salts of chloric acid. Other oxyanions of chlorine can be named "chlorate" followed by a Roman numeral in parentheses denoting the oxidation state of chlorine: e.g., the ClO−
4 ion commonly called perchlorate can also be called chlorate(VII).

In electrochemistry, cyclic voltammetry (CV) is a type of potentiodynamic measurement. In a cyclic voltammetry experiment, the working electrode potential is ramped linearly versus time. Unlike in linear sweep voltammetry, after the set potential is reached in a CV experiment, the working electrode's potential is ramped in the opposite direction to return to the initial potential. These cycles of ramps in potential may be repeated as many times as needed. The current at the working electrode is plotted versus the applied voltage to give the cyclic voltammogram trace. Cyclic voltammetry is generally used to study the electrochemical properties of an analyte in solution or of a molecule that is adsorbed onto the electrode.

Pitting corrosion, or pitting, is a form of extremely localized corrosion that leads to the random creation of small holes in metal. The driving power for pitting corrosion is the depassivation of a small area, which becomes anodic while an unknown but potentially vast area becomes cathodic, leading to very localized galvanic corrosion. The corrosion penetrates the mass of the metal, with a limited diffusion of ions.
Redox potential is a measure of the tendency of a chemical species to acquire electrons from or lose electrons to an electrode and thereby be reduced or oxidised respectively. Redox potential is expressed in volts (V). Each species has its own intrinsic redox potential; for example, the more positive the reduction potential, the greater the species' affinity for electrons and tendency to be reduced.

In electrochemistry, a salt bridge or ion bridge is an essential laboratory device discovered over 100 years ago. It contains an electrolyte solution, typically an inert solution, used to connect the oxidation and reduction half-cells of a galvanic cell, a type of electrochemical cell. In short, it functions as a link connecting the anode and cathode half-cells within an electrochemical cell. It also maintains electrical neutrality within the internal circuit and stabilizes the junction potential between the solutions in the half-cells. Additionally, it serves to minimize cross-contamination between the two half cells and helps concentrate our focus on unfolding the function of working electrodes of the half-cells.

A flow battery, or redox flow battery, is a type of electrochemical cell where chemical energy is provided by two chemical components dissolved in liquids that are pumped through the system on separate sides and in opposite direction of a membrane. Ion transfer inside the cell occurs through the membrane while both liquids circulate in their own respective space. Cell voltage is chemically determined by the Nernst equation and ranges, in practical applications, from 1.0 to 2.43 volts. The energy capacity is a function of the electrolyte volume and the power is a function of the surface area of the electrodes.
In electrochemistry, cell notation or cell representation is a shorthand method of expressing a reaction in an electrochemical cell.
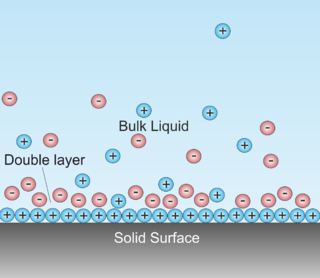
In surface science, a double layer is a structure that appears on the surface of an object when it is exposed to a fluid. The object might be a solid particle, a gas bubble, a liquid droplet, or a porous body. The DL refers to two parallel layers of charge surrounding the object. The first layer, the surface charge, consists of ions which are adsorbed onto the object due to chemical interactions. The second layer is composed of ions attracted to the surface charge via the Coulomb force, electrically screening the first layer. This second layer is loosely associated with the object. It is made of free ions that move in the fluid under the influence of electric attraction and thermal motion rather than being firmly anchored. It is thus called the "diffuse layer".
In chemistry, ion transport number, also called the transference number, is the fraction of the total electric current carried in an electrolyte by a given ionic species i:
The Iron Redox Flow Battery (IRFB), also known as Iron Salt Battery (ISB), stores and releases energy through the electrochemical reaction of iron salt. This type of battery belongs to the class of redox-flow batteries (RFB), which are alternative solutions to Lithium-Ion Batteries (LIB) for stationary applications. The IRFB can achieve up to 70% round trip energy efficiency. In comparison, other long duration storage technologies such as pumped hydro energy storage provide around 80% round trip energy efficiency.
References
- ↑ IUPAC , Compendium of Chemical Terminology , 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) " supporting electrolyte ". doi : 10.1351/goldbook.S06149
- ↑ Joseph Wang, "Analytical Electrochemistry", 3rd edition, Wiley VCH. 2006, ISBN 978-0-471-67879-3, p. 118.