
An oncogene is a gene that has the potential to cause cancer. In tumor cells, these genes are often mutated, or expressed at high levels.
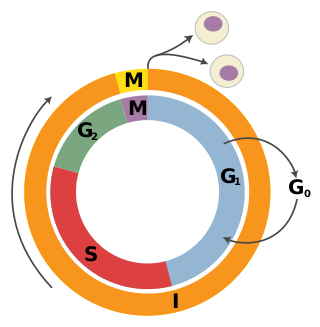
A tumor suppressor gene (TSG), or anti-oncogene, is a gene that regulates a cell during cell division and replication. If the cell grows uncontrollably, it will result in cancer. When a tumor suppressor gene is mutated, it results in a loss or reduction in its function. In combination with other genetic mutations, this could allow the cell to grow abnormally. The loss of function for these genes may be even more significant in the development of human cancers, compared to the activation of oncogenes.

BRCA2 and BRCA2 are human genes and their protein products, respectively. The official symbol and the official name are maintained by the HUGO Gene Nomenclature Committee. One alternative symbol, FANCD1, recognizes its association with the FANC protein complex. Orthologs, styled Brca2 and Brca2, are common in other vertebrate species. BRCA2 is a human tumor suppressor gene, found in all humans; its protein, also called by the synonym breast cancer type 2 susceptibility protein, is responsible for repairing DNA.
Chromosome 6 is one of the 23 pairs of chromosomes in humans. People normally have two copies of this chromosome. Chromosome 6 spans nearly 171 million base pairs and represents between 5.5 and 6% of the total DNA in cells. It contains the major histocompatibility complex, which contains over 100 genes related to the immune response, and plays a vital role in organ transplantation.

AKT2, also known as RAC-beta serine/threonine-protein kinase, is an enzyme that in humans is encoded by the AKT2 gene. It influences metabolite storage as part of the insulin signal transduction pathway.

NRAS is an enzyme that in humans is encoded by the NRAS gene. It was discovered by a small team of researchers led by Robin Weiss at the Institute of Cancer Research in London. It was the third RAS gene to be discovered, and was named NRAS, for its initial identification in human neuroblastoma cells.

Polycomb complex protein BMI-1 also known as polycomb group RING finger protein 4 (PCGF4) or RING finger protein 51 (RNF51) is a protein that in humans is encoded by the BMI1 gene. BMI1 is a polycomb ring finger oncogene.

Homeobox protein Hox-A9 is a protein that in humans is encoded by the HOXA9 gene.
Ras-related C3 botulinum toxin substrate 3 (Rac3) is a G protein that in humans is encoded by the RAC3 gene. It is an important component of intracellular signalling pathways. Rac3 is a member of the Rac subfamily of the Rho family of small G proteins. Members of this superfamily appear to regulate a diverse array of cellular events, including the control of cell growth, cytoskeletal reorganization, and the activation of protein kinases.

Fos-related antigen 1 (FRA1) is a protein that in humans is encoded by the FOSL1 gene.

H19 is a gene for a long noncoding RNA, found in humans and elsewhere. H19 has a role in the negative regulation of body weight and cell proliferation. This gene also has a role in the formation of some cancers and in the regulation of gene expression.

Elongation factor 1-alpha 2 is a protein that in humans is encoded by the EEF1A2 gene.

Suppressor of tumorigenicity protein 7 is a protein that in humans is encoded by the ST7 gene. ST7 orthologs have been identified in all mammals for which complete genome data are available.

ID4 is a protein coding gene. In humans, it encodes the protein known as DNA-binding protein inhibitor ID-4. This protein is known to be involved in the regulation of many cellular processes during both prenatal development and tumorigenesis. This is inclusive of embryonic cellular growth, senescence, cellular differentiation, apoptosis, and as an oncogene in angiogenesis.

CDKN2A, also known as cyclin-dependent kinase inhibitor 2A, is a gene which in humans is located at chromosome 9, band p21.3. It is ubiquitously expressed in many tissues and cell types. The gene codes for two proteins, including the INK4 family member p16 and p14arf. Both act as tumor suppressors by regulating the cell cycle. p16 inhibits cyclin dependent kinases 4 and 6 and thereby activates the retinoblastoma (Rb) family of proteins, which block traversal from G1 to S-phase. p14ARF activates the p53 tumor suppressor. Somatic mutations of CDKN2A are common in the majority of human cancers, with estimates that CDKN2A is the second most commonly inactivated gene in cancer after p53. Germline mutations of CDKN2A are associated with familial melanoma, glioblastoma and pancreatic cancer. The CDKN2A gene also contains one of 27 SNPs associated with increased risk of coronary artery disease.
A metastasis suppressor is a protein that acts to slow or prevent metastases from spreading in the body of an organism with cancer. Metastasis is one of the most lethal cancer processes. This process is responsible for about ninety percent of human cancer deaths. Proteins that act to slow or prevent metastases are different from those that act to suppress tumor growth. Genes for about a dozen such proteins are known in humans and other animals.

Suppression of tumorigenicity 5 is a protein that in humans is encoded by the ST5 gene. ST5 orthologs have been identified in nearly all mammals for which complete genome data are available.

Ribonuclease T2 is an enzyme that in humans is encoded by the RNASET2 gene. It is a type of endoribonuclease.

MCTS1, re-initiation and release factor, otherwise known as MCT-1, is a protein that in humans is encoded by the MCTS1 gene.














