
Magnetic resonance imaging (MRI) is a medical imaging technique used in radiology to form pictures of the anatomy and the physiological processes of the body. MRI scanners use strong magnetic fields, magnetic field gradients, and radio waves to generate images of the organs in the body. MRI does not involve X-rays or the use of ionizing radiation, which distinguishes it from computed tomography (CT) and positron emission tomography (PET) scans. MRI is a medical application of nuclear magnetic resonance (NMR) which can also be used for imaging in other NMR applications, such as NMR spectroscopy.

Functional magnetic resonance imaging or functional MRI (fMRI) measures brain activity by detecting changes associated with blood flow. This technique relies on the fact that cerebral blood flow and neuronal activation are coupled. When an area of the brain is in use, blood flow to that region also increases.
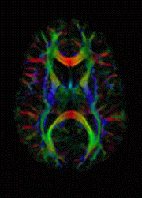
Diffusion-weighted magnetic resonance imaging is the use of specific MRI sequences as well as software that generates images from the resulting data that uses the diffusion of water molecules to generate contrast in MR images. It allows the mapping of the diffusion process of molecules, mainly water, in biological tissues, in vivo and non-invasively. Molecular diffusion in tissues is not random, but reflects interactions with many obstacles, such as macromolecules, fibers, and membranes. Water molecule diffusion patterns can therefore reveal microscopic details about tissue architecture, either normal or in a diseased state. A special kind of DWI, diffusion tensor imaging (DTI), has been used extensively to map white matter tractography in the brain.

Magnetic resonance angiography (MRA) is a group of techniques based on magnetic resonance imaging (MRI) to image blood vessels. Magnetic resonance angiography is used to generate images of arteries in order to evaluate them for stenosis, occlusions, aneurysms or other abnormalities. MRA is often used to evaluate the arteries of the neck and brain, the thoracic and abdominal aorta, the renal arteries, and the legs.
In MRI and NMR spectroscopy, an observable nuclear spin polarization (magnetization) is created by a homogeneous magnetic field. This field makes the magnetic dipole moments of the sample precess at the resonance (Larmor) frequency of the nuclei. At thermal equilibrium, nuclear spins precess randomly about the direction of the applied field. They become abruptly phase coherent when they are hit by radiofrequency (RF) pulses at the resonant frequency, created orthogonal to the field. The RF pulses cause the population of spin-states to be perturbed from their thermal equilibrium value. The generated transverse magnetization can then induce a signal in an RF coil that can be detected and amplified by an RF receiver. The return of the longitudinal component of the magnetization to its equilibrium value is termed spin-latticerelaxation while the loss of phase-coherence of the spins is termed spin-spin relaxation, which is manifest as an observed free induction decay (FID).
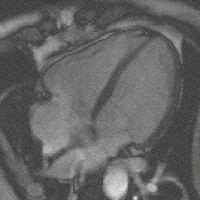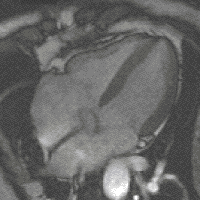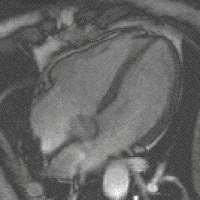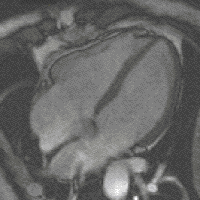
Steady-state free precession (SSFP) imaging is a magnetic resonance imaging (MRI) sequence which uses steady states of magnetizations. In general, SSFP MRI sequences are based on a gradient echo MRI sequence with a short repetition time which in its generic form has been described as the FLASH MRI technique. While spoiled gradient-echo sequences refer to a steady state of the longitudinal magnetization only, SSFP gradient-echo sequences include transverse coherences (magnetizations) from overlapping multi-order spin echoes and stimulated echoes. This is usually accomplished by refocusing the phase-encoding gradient in each repetition interval in order to keep the phase integral constant. Fully balanced SSFP MRI sequences achieve a phase of zero by refocusing all imaging gradients.
Fast low angle shot magnetic resonance imaging is a particular sequence of magnetic resonance imaging. It is a gradient echo sequence which combines a low-flip angle radio-frequency excitation of the nuclear magnetic resonance signal with a short repetition time. It is the generic form of steady-state free precession imaging.

Fluid-attenuated inversion recovery (FLAIR) is an MRI sequence with an inversion recovery set to null fluids. For example, it can be used in brain imaging to suppress cerebrospinal fluid (CSF) effects on the image, so as to bring out the periventricular hyperintense lesions, such as multiple sclerosis (MS) plaques. It was invented by Graeme Bydder. FLAIR can be used with both three-dimensional imaging or two dimensional imaging.

In physics, the spin–spin relaxation is the mechanism by which Mxy, the transverse component of the magnetization vector, exponentially decays towards its equilibrium value in nuclear magnetic resonance (NMR) and magnetic resonance imaging (MRI). It is characterized by the spin–spin relaxation time, known as T2, a time constant characterizing the signal decay. It is named in contrast to T1, the spin–lattice relaxation time. It is the time it takes for the magnetic resonance signal to irreversibly decay to 37% (1/e) of its initial value after its generation by tipping the longitudinal magnetization towards the magnetic transverse plane. Hence the relation
During nuclear magnetic resonance observations, spin–lattice relaxation is the mechanism by which the longitudinal component of the total nuclear magnetic moment vector (parallel to the constant magnetic field) exponentially relaxes from a higher energy, non-equilibrium state to thermodynamic equilibrium with its surroundings (the "lattice"). It is characterized by the spin–lattice relaxation time, a time constant known as T1.

The physics of magnetic resonance imaging (MRI) concerns fundamental physical considerations of MRI techniques and technological aspects of MRI devices. MRI is a medical imaging technique mostly used in radiology and nuclear medicine in order to investigate the anatomy and physiology of the body, and to detect pathologies including tumors, inflammation, neurological conditions such as stroke, disorders of muscles and joints, and abnormalities in the heart and blood vessels among others. Contrast agents may be injected intravenously or into a joint to enhance the image and facilitate diagnosis. Unlike CT and X-ray, MRI uses no ionizing radiation and is, therefore, a safe procedure suitable for diagnosis in children and repeated runs. Patients with specific non-ferromagnetic metal implants, cochlear implants, and cardiac pacemakers nowadays may also have an MRI in spite of effects of the strong magnetic fields. This does not apply on older devices, and details for medical professionals are provided by the device's manufacturer.

Real-time magnetic resonance imaging (RT-MRI) refers to the continuous monitoring ("filming") of moving objects in real time. Because MRI is based on time-consuming scanning of k-space, real-time MRI was possible only with low image quality or low temporal resolution. Using an iterative reconstruction algorithm these limitations have recently been removed: a new method for real-time MRI achieves a temporal resolution of 20 to 30 milliseconds for images with an in-plane resolution of 1.5 to 2.0 mm. Real-time MRI promises to add important information about diseases of the joints and the heart. In many cases MRI examinations may become easier and more comfortable for patients.

Quantitative susceptibility mapping (QSM) provides a novel contrast mechanism in magnetic resonance imaging (MRI) different from traditional susceptibility weighted imaging. The voxel intensity in QSM is linearly proportional to the underlying tissue apparent magnetic susceptibility, which is useful for chemical identification and quantification of specific biomarkers including iron, calcium, gadolinium, and super paramagnetic iron oxide (SPIO) nano-particles. QSM utilizes phase images, solves the magnetic field to susceptibility source inverse problem, and generates a three-dimensional susceptibility distribution. Due to its quantitative nature and sensitivity to certain kinds of material, potential QSM applications include standardized quantitative stratification of cerebral microbleeds and neurodegenerative disease, accurate gadolinium quantification in contrast enhanced MRI, and direct monitoring of targeted theranostic drug biodistribution in nanomedicine.

Magnetic resonance imaging of the brain uses magnetic resonance imaging (MRI) to produce high quality two-dimensional or three-dimensional images of the brain and brainstem as well as the cerebellum without the use of ionizing radiation (X-rays) or radioactive tracers.

Perfusion MRI or perfusion-weighted imaging (PWI) is perfusion scanning by the use of a particular MRI sequence. The acquired data are then post-processed to obtain perfusion maps with different parameters, such as BV, BF, MTT and TTP.

An MRI sequence in magnetic resonance imaging (MRI) is a particular setting of pulse sequences and pulsed field gradients, resulting in a particular image appearance.
Gradient echo is a magnetic resonance imaging (MRI) sequence that has wide variety of applications, from magnetic resonance angiography to perfusion MRI and diffusion MRI. Rapid imaging acquisition allows it to be applied to 2D and 3D MRI imaging. Gradient echo uses magnetic gradients to generate a signal, instead of using 180 degrees radiofrequency pulse like spin echo; thus leading to faster image acquisition time.
An MRI artifact is a visual artifact in magnetic resonance imaging (MRI). It is a feature appearing in an image that is not present in the original object. Many different artifacts can occur during MRI, some affecting the diagnostic quality, while others may be confused with pathology. Artifacts can be classified as patient-related, signal processing-dependent and hardware (machine)-related.
Inversion recovery is an MRI sequence that provides high contrast between tissue and lesion. It can be used to provide high T1 weighted image, high T2 weighted image, and to suppress the signals from fat, blood, or cerebrospinal fluid (CSF).
Magnetic resonance fingerprinting (MRF) is methodology in quantitative magnetic resonance imaging (MRI) characterized by a pseudo-randomized acquisition strategy. It involves creating unique signal patterns or 'fingerprints' for different materials or tissues after which a pattern recognition algorithm matches these fingerprints with a predefined dictionary of expected signal patterns. This process translates the data into quantitative maps, revealing information about the magnetic properties being investigated.




















