Related Research Articles
Chemical synthesis is the artificial execution of useful chemical reactions to obtain one or several products. This occurs by physical and chemical manipulations usually involving one or more reactions. In modern laboratory uses, the process is reproducible, reliable, and established to work the same in multiple laboratories.
In organic chemistry, the Diels–Alder reaction is a chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene derivative. It is the prototypical example of a pericyclic reaction with a concerted mechanism. More specifically, it is classified as a thermally-allowed [4+2] cycloaddition with Woodward–Hoffmann symbol [π4s + π2s]. It was first described by Otto Diels and Kurt Alder in 1928. For the discovery of this reaction, they were awarded the Nobel Prize in Chemistry in 1950. Through the simultaneous construction of two new carbon–carbon bonds, the Diels–Alder reaction provides a reliable way to form six-membered rings with good control over the regio- and stereochemical outcomes. Consequently, it has served as a powerful and widely applied tool for the introduction of chemical complexity in the synthesis of natural products and new materials. The underlying concept has also been applied to π-systems involving heteroatoms, such as carbonyls and imines, which furnish the corresponding heterocycles; this variant is known as the hetero-Diels–Alder reaction. The reaction has also been generalized to other ring sizes, although none of these generalizations have matched the formation of six-membered rings in terms of scope or versatility. Because of the negative values of ΔH° and ΔS° for a typical Diels–Alder reaction, the microscopic reverse of a Diels–Alder reactions becomes favorable at high temperatures, although this is of synthetic importance for only a limited range of Diels-Alder adducts, generally with some special structural features; this reverse reaction is known as the retro-Diels–Alder reaction.
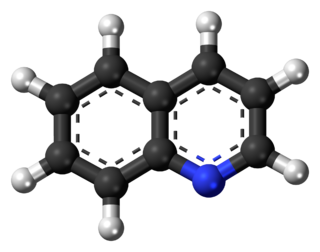
Quinoline is a heterocyclic aromatic organic compound with the chemical formula C9H7N. It is a colorless hygroscopic liquid with a strong odor. Aged samples, especially if exposed to light, become yellow and later brown. Quinoline is only slightly soluble in cold water but dissolves readily in hot water and most organic solvents. Quinoline itself has few applications, but many of its derivatives are useful in diverse applications. A prominent example is quinine, an alkaloid found in plants. 4-Hydroxy-2-alkylquinolines (HAQs) are involved in antibiotic resistance.
The Friedel–Crafts reactions are a set of reactions developed by Charles Friedel and James Crafts in 1877 to attach substituents to an aromatic ring. Friedel–Crafts reactions are of two main types: alkylation reactions and acylation reactions. Both proceed by electrophilic aromatic substitution.

An imine is a functional group or chemical compound containing a carbon–nitrogen double bond. The nitrogen atom can be attached to a hydrogen (H) or an organic group (R). If this group is not a hydrogen atom, then the compound can sometimes be referred to as a Schiff base. The carbon atom has two additional single bonds. The term "imine" was coined in 1883 by the German chemist Albert Ladenburg.

William Alfred Fowler was an American nuclear physicist, later astrophysicist, who, with Subrahmanyan Chandrasekhar won the 1983 Nobel Prize in Physics. He is known for his theoretical and experimental research into nuclear reactions within stars and the energy elements produced in the process.
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one of the most important branches of organic chemistry. There are several main areas of research within the general area of organic synthesis: total synthesis, semisynthesis, and methodology.

Pyridinium chlorochromate (PCC) is a yellow-orange salt with the formula [C5H5NH]+[CrO3Cl]−. It is a reagent in organic synthesis used primarily for oxidation of alcohols to form carbonyls. A variety of related compounds are known with similar reactivity. PCC offers the advantage of the selective oxidation of alcohols to aldehydes or ketones, whereas many other reagents are less selective.
In chemistry, a dehydration reaction is a conversion that involves the loss of water from the reacting molecule or ion. Dehydration reactions are common processes, the reverse of a hydration reaction. Common dehydrating agents used in organic synthesis include sulfuric acid and alumina. Often dehydration reactions are effected with heating.
In chemistry a divergent synthesis is a strategy with the aim to improve the efficiency of chemical synthesis. It is often an alternative to convergent synthesis or linear synthesis.
A coupling reaction in organic chemistry is a general term for a variety of reactions where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M reacts with an organic halide of the type R'-X with formation of a new carbon-carbon bond in the product R-R'. The most common type of coupling reaction is the cross coupling reaction.
The Mannich reaction is an organic reaction which consists of an amino alkylation of an acidic proton placed next to a carbonyl functional group by formaldehyde and a primary or secondary amine or ammonia. The final product is a β-amino-carbonyl compound also known as a Mannich base. Reactions between aldimines and α-methylene carbonyls are also considered Mannich reactions because these imines form between amines and aldehydes. The reaction is named after chemist Carl Mannich.
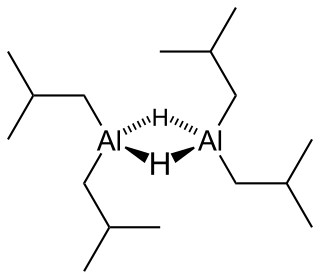
Diisobutylaluminium hydride (DIBALH, DIBAL, DIBAL-H or DIBAH, DY-bal) is a reducing agent with the formula (i-Bu2AlH)2, where i-Bu represents isobutyl (-CH2CH(CH3)2). This organoaluminium compound was investigated originally as a co-catalyst for the polymerization of alkenes.
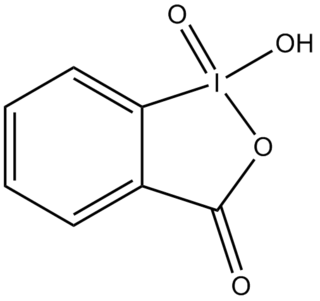
2-Iodoxybenzoic acid (IBX) is an organic compound used in organic synthesis as an oxidizing agent. This periodinane is especially suited to oxidize alcohols to aldehydes. IBX is prepared from 2-iodobenzoic acid, potassium bromate, and sulfuric acid. Frigerio and co-workers have also demonstrated, in 1999 that potassium bromate may be replaced by commercially available Oxone. One of the main drawbacks of IBX is its limited solubility; IBX is insoluble in many common organic solvents. In the past, it was believed that IBX was shock sensitive, but it was later proposed that samples of IBX were shock sensitive due to the residual potassium bromate left from its preparation. Commercial IBX is stabilized by carboxylic acids such as benzoic acid and isophthalic acid.
Organosilicon compounds are organometallic compounds containing carbon–silicon bonds. Organosilicon chemistry is the corresponding science of their preparation and properties. Most organosilicon compounds are similar to the ordinary organic compounds, being colourless, flammable, hydrophobic, and stable to air. Silicon carbide is an inorganic compound.
Oppenauer oxidation, named after Rupert Viktor Oppenauer, is a gentle method for selectively oxidizing secondary alcohols to ketones.

Stephen F. Martin is an American chemist and professor of chemistry at The University of Texas at Austin. He is the M. June and J. Virgil Waggoner Regents Chair in Chemistry.

Richard Frederick Heck was an American chemist noted for the discovery and development of the Heck reaction, which uses palladium to catalyze organic chemical reactions that couple aryl halides with alkenes. The analgesic naproxen is an example of a compound that is prepared industrially using the Heck reaction.
In organophosphorus chemistry, the Kabachnik–Fields reaction is a three-component organic reaction forming α-aminomethylphosphonates from an amine, a carbonyl compound, and a dialkyl phosphonate, (RO)2P(O)H (that are also called dialkylphosphites). Aminophosphonates are synthetic targets of some importance as phosphorus analogues of α-amino acids (a bioisostere). This multicomponent reaction was independently discovered by Martin Izrailevich Kabachnik and Ellis K. Fields in 1952. The reaction is very similar to the two-component Pudovik reaction, which involves condensation of the phosphite and a preformed imine.
References
- ↑ Martin, S. F.; Sunderhaus, J. D.; Dockendorff, C. (2007). "Applications of Multicomponent Reactions for the Synthesis of Diverse Heterocyclic Scaffolds". Org. Lett. 9 (21): 4223–6. doi:10.1021/ol7018357. PMC 2803615 . PMID 17887692.