Charybdotoxin (CTX) is a 37 amino acid neurotoxin from the venom of the scorpion Leiurus quinquestriatus hebraeus (deathstalker) that blocks calcium-activated potassium channels. This blockade causes hyperexcitability of the nervous system. It is a close homologue of agitoxin and both toxins come from Leiurus quinquestriatus hebraeus.
Omega-grammotoxin SIA is a protein toxin that inhibits P, Q and N voltage-gated calcium channels (Ca2+ channels) in neurons.
Taicatoxin (TCX) is a snake toxin that blocks voltage-dependent L-type calcium channels and small conductance Ca2+-activated K+ channels. The name taicatoxin (TAIpan + CAlcium + TOXIN) is derived from its natural source, the taipan snake, the site of its action, calcium channels, and from its function as a toxin. Taicatoxin was isolated from the venom of Australian taipan snake, Oxyuranus scutellatus scutellatus. TCX is a secreted protein, produced in the venom gland of the snake.
Tityustoxin is a toxin found in the venom of scorpions from the subfamily of Tityinae. By binding to voltage-dependent sodium ion channels and potassium channels, they cause sialorrhea, lacrimation and rhinorrhea.

Slotoxin is a peptide from Centruroides noxius Hoffmann scorpion venom. It belongs to the short scorpion toxin superfamily.
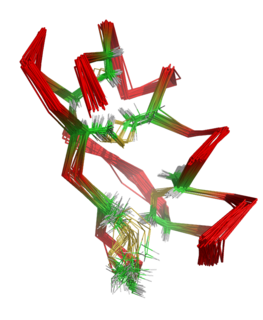
Maurotoxin is a peptide toxin from the venom of the Tunisian chactoid scorpion Scorpio maurus palmatus, from which it was first isolated and from which the chemical gets its name. It acts by blocking several types of voltage-gated potassium channel.
Iberiotoxin (IbTX) is an ion channel toxin purified from the Eastern Indian red scorpion Buthus tamulus. Iberiotoxin selectively inhibits the current through large-conductance calcium-activated potassium channels.
Phoneutria nigriventer toxin-3 is more commonly referred to as PhTx3.
In molecular biology, the BmKK2 toxins are a family of scorpion toxins. They belong to the scorpion toxin subfamily alpha-KTx 14. They include a novel short-chain peptide from the Asian scorpion Mesobuthus martensii Karsch, a potassium channel blocker composed of 31 amino acid residues. The peptide adopts a classical alpha/beta-scaffold for alpha-KTxs. BmKK2 selectively inhibits the delayed rectifier K+ current, but does not affect the fast transient K+ current.
BeKm-1 is a toxin from the Central Asian scorpion Buthus eupeus. BeKm-1 acts by selectively inhibiting the human Ether-à-go-go Related Gene (hERG) channels, which are voltage gated potassium ion channels.

Pi3 toxin is a purified peptide derivative of the Pandinus imperator scorpion venom. It is a potent blocker of voltage-gated potassium channel, Kv1.3 and is closely related to another peptide found in the venom, Pi2.
Tamulotoxin is a venomous neurotoxin from the Indian Red Scorpion.
HsTx1 is a toxin from the venom of the scorpion Heterometrus spinifer. HsTx1 is a very potent inhibitor of the rat Kv1.3 voltage-gated potassium channel.
Pi4 is a short toxin from the scorpion Pandinus imperator that blocks specific potassium channels.
Limbatustoxin, is an ion channel toxin from the venom of the Centruroides limbatus scorpion. This toxin is a selective blocker of BK channels, calcium-activated potassium channels.
Tityustoxin peptide 2 (TsPep2) is a peptide isolated from the venom of the Tityus serrulatus. It belongs to a class of short peptides, together with Tityustoxin peptide 1 and Tityustoxin peptide 3.

Noxiustoxin (NTX) is a toxin from the venom of the Mexican scorpion Centruroides noxius Hoffmann which block voltage-dependent potassium channels and calcium-activated potassium channels.

BmP02, also known as α-KTx 9.1 or Bmkk(6), is a toxin from the Buthus Martensi Karsch (BmK) scorpion. The toxin acts on potassium channels, blocking Kv1.3 and slowing the deactivation of Kv4.2. BmP02 is not toxic to humans or mice.