Immunotherapy or biological therapy is the treatment of disease by activating or suppressing the immune system. Immunotherapies designed to elicit or amplify an immune response are classified as activation immunotherapies, while immunotherapies that reduce or suppress are classified as suppression immunotherapies.

Tumor necrosis factor is a cytokine, i.e. a small protein used by the immune system for cell signaling. If macrophages detect an infection, they release TNF in order to alert other cells of the immune system as well as cells of other tissues, leading to inflammation. TNF is a member of the TNF superfamily, which consists of various transmembrane proteins with a homologous TNF domain.
Cytokine release syndrome (CRS) is a form of systemic inflammatory response syndrome (SIRS) that can be triggered by a variety of factors such as infections and certain drugs. It refers to cytokine storm syndromes (CSS) and occurs when large numbers of white blood cells are activated and release inflammatory cytokines, which in turn activate yet more white blood cells. CRS is also an adverse effect of some monoclonal antibody medications, as well as adoptive T-cell therapies. When occurring as a result of a medication, it is also known as an infusion reaction.

Gingerol, properly as [6]-gingerol, is a phenol phytochemical compound found in fresh ginger that activates spice receptors on the tongue. Molecularly, gingerol is a relative of capsaicin and piperine, the compounds which are alkaloids, though the bioactive pathways are unconnected. It is normally found as a pungent yellow oil in the ginger rhizome, but can also form a low-melting crystalline solid. This chemical compound is found in all members of the Zingiberaceae family plant and is high in concentrations in the grains of paradise as well as an African Ginger species.

Phorbol is a natural, plant-derived organic compound. It is a member of the tigliane family of diterpenes. Phorbol was first isolated in 1934 as the hydrolysis product of croton oil, which is derived from the seeds of the purging croton, Croton tiglium. The structure of phorbol was determined in 1967. Various esters of phorbol have important biological properties, the most notable of which is the capacity to act as tumor promoters through activation of protein kinase C. They mimic diacylglycerols, glycerol derivatives in which two hydroxyl groups have reacted with fatty acids to form esters. The most common and potent phorbol ester is 12-O-tetradecanoylphorbol-13-acetate (TPA), also called phorbol-12-myristate-13-acetate (PMA), which is used as a biomedical research tool in contexts such as models of carcinogenesis.
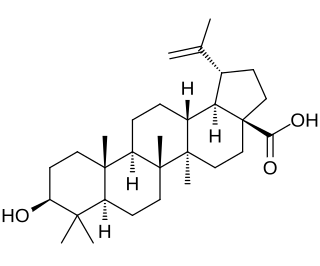
Betulinic acid is a naturally occurring pentacyclic triterpenoid which has antiretroviral, antimalarial, and anti-inflammatory properties, as well as a more recently discovered potential as an anticancer agent, by inhibition of topoisomerase. It is found in the bark of several species of plants, principally the white birch from which it gets its name, but also the ber tree, selfheal, the tropical carnivorous plants Triphyophyllum peltatum and Ancistrocladus heyneanus, Diospyros leucomelas, a member of the persimmon family, Tetracera boiviniana, the jambul, flowering quince, rosemary, and Pulsatilla chinensis.

Salvia miltiorrhiza, also known as red sage, Chinese sage, tan shen, or danshen, is a perennial plant in the genus Salvia, highly valued for its roots in traditional Chinese medicine. Native to China and Japan, it grows at 90 to 1,200 m elevation, preferring grassy places in forests, hillsides, and along stream banks. The specific epithet miltiorrhiza means "red ochre root". Since the outbreak of SARS in 2003, this herb has been tested for treatment of COPD with promising results.

Helenalin, or (-)-4-Hydroxy-4a,8-dimethyl-3,3a,4a,7a,8,9,9a-octahydroazuleno[6,5-b]furan-2,5-dione, is a toxic sesquiterpene lactone which can be found in several plants such as Arnica montana and Arnica chamissonis subsp. foliosa. Helenalin is responsible for the toxicity of the Arnica spp. Although toxic, helenalin possesses some in vitro anti-inflammatory and anti-neoplastic effects. Helenalin can inhibit certain enzymes, such as 5-lipoxygenase and leukotriene C4 synthase. For this reason the compound or its derivatives may have potential medical applications.
Sodium salicylate is a sodium salt of salicylic acid. It can be prepared from sodium phenolate and carbon dioxide under higher temperature and pressure. Historically, it has been synthesized by refluxing methyl salicylate with an excess of sodium hydroxide.
Histone deacetylase inhibitors are chemical compounds that inhibit histone deacetylases.

Protocatechuic acid (PCA) is a dihydroxybenzoic acid, a type of phenolic acid. It is a major metabolite of antioxidant polyphenols found in green tea. It has mixed effects on normal and cancer cells in in vitro and in vivo studies.

Lithocholic acid, also known as 3α-hydroxy-5β-cholan-24-oic acid or LCA, is a bile acid that acts as a detergent to solubilize fats for absorption. Bacterial action in the colon produces LCA from chenodeoxycholic acid by reduction of the hydroxyl functional group at carbon-7 in the "B" ring of the steroid framework.

A trifunctional antibody is a monoclonal antibody with binding sites for two different antigens, typically CD3 and a tumor antigen, making it a type of bispecific monoclonal antibody. In addition, its intact Fc-part can bind to an Fc receptor on accessory cells like conventional monospecific antibodies. The net effect is that this type of drug links T cells and monocytes/macrophages, natural killer cells, dendritic cells or other Fc receptor expressing cells to the tumor cells, leading to their destruction.

Deacetylasperulosidic acid is an iridoid compound found in a few medicinal plants, such as Morinda citrifolia. Some in vitro and in vivo bioactivities of deacetylasperulosidic acid include anti-inflammatory, analgesic, anti-cancer, antioxidant, anti-arthritic, anti-mutagenic, anti-clastogenic, and hepatoprotection.

Polyporus umbellatus is a rare, edible species of mushroom, found growing on roots of old beeches or oak (e.g.). It is also called lumpy bracket and umbrella polypore.

A quinone methide is a type of conjugated organic compound that contain a cyclohexadiene with a carbonyl and an exocyclic methylidene or extended alkene unit. It is analogous to a quinone, but having one of the double bonded oxygens replaced with a carbon. The carbonyl and methylidene are usually oriented either ortho or para to each other. There are some examples of transient synthetic meta quinone methides.
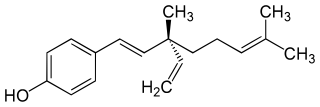
Bakuchiol is a meroterpene in the class terpenophenol.

Withaferin A is a steroidal lactone, derived from Acnistus arborescens, Withania somnifera and other members of family Solanaceae. It has been traditionally used in ayurvedic medicine. It is the first member of the withanolide class of ergostane type product to be discovered. This natural product has wide range of pharmacological activities including cardioprotective, anti-inflammatory, immuno-modulatory, anti-angiogenesis, anti-metastasis and anti-carcinogenic properties.

Carnosol is a phenolic diterpene found in the herbs rosemary and Mountain desert sage.

Cucurbitacin E is a biochemical compound from the family of cucurbitacins. These are found in plants which are member of the family Cucurbitaceae, most of them coming from traditional Chinese medicinal plants, but also in other plants such as pumpkins and gourds.
















