Related Research Articles

Infrared spectroscopy is the measurement of the interaction of infrared radiation with matter by absorption, emission, or reflection. It is used to study and identify chemical substances or functional groups in solid, liquid, or gaseous forms. It can be used to characterize new materials or identify and verify known and unknown samples. The method or technique of infrared spectroscopy is conducted with an instrument called an infrared spectrometer which produces an infrared spectrum. An IR spectrum can be visualized in a graph of infrared light absorbance on the vertical axis vs. frequency, wavenumber or wavelength on the horizontal axis. Typical units of wavenumber used in IR spectra are reciprocal centimeters, with the symbol cm−1. Units of IR wavelength are commonly given in micrometers, symbol μm, which are related to the wavenumber in a reciprocal way. A common laboratory instrument that uses this technique is a Fourier transform infrared (FTIR) spectrometer. Two-dimensional IR is also possible as discussed below.

Spectroscopy is the field of study that measures and interprets electromagnetic spectra. In narrower contexts, spectroscopy is the precise study of color as generalized from visible light to all bands of the electromagnetic spectrum.

Raman spectroscopy is a spectroscopic technique typically used to determine vibrational modes of molecules, although rotational and other low-frequency modes of systems may also be observed. Raman spectroscopy is commonly used in chemistry to provide a structural fingerprint by which molecules can be identified.
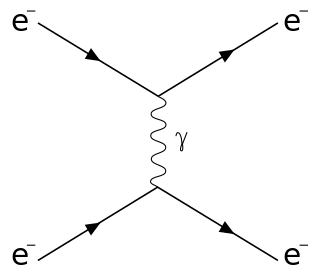
In physics, Raman scattering or the Raman effect is the inelastic scattering of photons by matter, meaning that there is both an exchange of energy and a change in the light's direction. Typically this effect involves vibrational energy being gained by a molecule as incident photons from a visible laser are shifted to lower energy. This is called normal Stokes-Raman scattering.
A molecular vibration is a periodic motion of the atoms of a molecule relative to each other, such that the center of mass of the molecule remains unchanged. The typical vibrational frequencies range from less than 1013 Hz to approximately 1014 Hz, corresponding to wavenumbers of approximately 300 to 3000 cm−1 and wavelengths of approximately 30 to 3 µm.

The absorption of electromagnetic radiation by water depends on the state of the water.
Eudialyte group is a group of complex trigonal zircono- and, more rarely, titanosilicate minerals with general formula [N(1)N(2)N(3)N(4)N(5)]3[M(1a)M(1b)]3M(2)3M(4)Z3[Si24O72]O'4X2, where N(1) and N(2) and N(3) and N(5) = Na+ and more rarely H3O+ or H2O, N(4) = Na+, Sr2+, Mn2+ and more rarely H3O+ or H2O or K+ or Ca2+ or REE3+ (rare earth elements), M(1) and M(1b) = Ca2+, M(1a) = Ca2+ or Mn2+ or Fe2+, M(2) = Fe (both II and III), Mn and rarely Na+, K+ or Zr4+, M(3) = Si, Nb and rarely W, Ti and [] (vacancy), M(4) = Si and or rarely [], Z Zr4+ and or rarely Ti4+, and X = OH−, Cl− and more rarely CO32− or F−. Some of the eudialyte-like structures can even be more complex, however, in general, its typical feature is the presence of [Si3O9]6− and [Si9O27]18− ring silicate groups. Space group is usually R3m or R-3m but may be reduced to R3 due to cation ordering. Like other zirconosilicates, the eudialyte group minerals possess alkaline ion-exchange properties, as microporous materials.
Vibronic spectroscopy is a branch of molecular spectroscopy concerned with vibronic transitions: the simultaneous changes in electronic and vibrational energy levels of a molecule due to the absorption or emission of a photon of the appropriate energy. In the gas phase, vibronic transitions are accompanied by changes in rotational energy also.
Brillouin spectroscopy is an empirical spectroscopy technique which allows the determination of elastic moduli of materials. The technique uses inelastic scattering of light when it encounters acoustic phonons in a crystal, a process known as Brillouin scattering, to determine phonon energies and therefore interatomic potentials of a material. The scattering occurs when an electromagnetic wave interacts with a density wave, photon-phonon scattering.

Ikranite is a member of the eudialyte group, named after the Shubinov Institute of Crystallography of the Russian Academy of Sciences. It is a cyclosilicate mineral that shows trigonal symmetry with the space group R3m, and is often seen with a pseudo-hexagonal habit. Ikranite appears as translucent and ranges in color from yellow to a brownish yellow. This mineral ranks a 5 on Mohs scale of mineral hardness, though it is considered brittle, exhibiting conchoidal fracture when broken.
Carbokentbrooksite is a very rare mineral of the eudialyte group, with formula (Na,□)12(Na,Ce)3Ca6Mn3Zr3NbSiO(Si9O27)2(Si3O9)2(OH)3(CO3).H2O. The original formula was extended to show the presence of cyclic silicate groups and silicon at the M4 site, according to the nomenclature of eudialyte group. Carbokenbrooksite characterizes in being carbonate-rich (the other eudialyte-group species with essential carbonate are zirsilite-(Ce), golyshevite, and mogovidite). It is also sodium rich, being sodium equivalent of zirsilite-(Ce), with which it is intimately associated.
Labyrinthite is a very rare mineral of the eudialyte group. When compared to other species in the group, its structure is extremely complex – with over 100 sites and about 800 cations and anions – hence its name, with its complexity expressed in its chemical formula (Na,K,Sr)35Ca12Fe3Zr6TiSi51O144(O,OH,H2O)9Cl3. The formula is simplified as it does not show the presence of cyclic silicate groups. Complexity of the structure results in symmetry lowering from the typical centrosymmetrical group to R3 space group. Other eudialyte-group representatives with such symmetry lowering include aqualite, oneillite, raslakite, voronkovite. Labyrinthite is the second dual-nature representative of the group after dualite and third with essential titanium after dualite and alluaivite.
Feklichevite is a rare mineral of the eudialyte group with the formula Na11Ca9(Fe3+,Fe2+)2Zr3NbSi(Si3O9)2(Si9O27)2. The original formula was extended to show the presence of cyclic silicate groups and presence of silicon at the M4 site, according to the nomenclature of eudialyte group. When compared to other minerals of the group, feklichevite characterizes in the presence of ferric iron (thus similar to ikranite, mogovidite and fengchengite) and dominance of calcium at the N4 site. Calcium is ordered in the structure and is also present at the M1 site. Other iron-bearing minerals of the group are eudialyte, ferrokentbrooksite, georgbarsanovite, khomyakovite, labyrinthite, oneillite and rastsvetaevite, but they rather contain ferrous iron Feklichevite name honors Russian mineralogist and crystallographer, V. G. Feklichev.

Golyshevite is a rare mineral of the eudialyte group, with the formula Na10Ca3Ca6Zr3Fe2SiNb(Si3O9)2(Si9O27)2CO3(OH)3•H2O. The original formula was extended to show both the presence of cyclic silicate groups and silicon at the M4 site, according to the nomenclature of the eudialyte group. The characteristic feature of golyshevite is calcium-rich composition, with calcium at two main sites instead of one site. Together with feklichevite, fengchengite, ikranite and mogovidite it is a ferric-iron-dominant representative of the group. It is chemically similar to mogovidite. Golyshevite was named after Russian crystallographer Vladimir Mikhailovich Golyshev.
Johnsenite-(Ce) is a very rare mineral of the eudialyte group, with the chemical formula Na12(Ce,La,Sr,Ca,[ ])3Ca6Mn3Zr3WSi(Si9O27)2(Si3O9)2(CO3)O(OH,Cl)2. The original formula was extended to show the presence of both the cyclic silicate groups and silicon at the M4 site, according to the nomenclature of the eudialyte group. It is the third eudialyte-group mineral with essential tungsten, and second with essential rare earth elements. In fact, some niobium substitutes for tungsten in johnsenite-(Ce). Other characteristic feature is the presence of essential carbonate group, shared with carbokentbrooksite, golyshevite, mogovidite and zirsilite-(Ce).

Khomyakovite is an exceedingly rare mineral of the eudialyte group, with formula Na12Sr3Ca6Fe3Zr3W(Si25O73)(O,OH,H2O)3(OH,Cl)2. The original formula was extended to show the presence of both the cyclic silicate groups and M4-site silicon, according to the nomenclature of the eudialyte group. Some niobium substitutes for tungsten in khomyakovite. Khomyakovite is an iron-analogue of manganokhomyakovite, the second mineral being a bit more common. The two minerals are the only group representatives, beside taseqite, with species-defining strontium, although many other members display strontium diadochy. Khomyakovite is the third eudialyte-group mineral with essential tungsten.

Manganokhomyakovite is a very rare mineral of the eudialyte group, with the chemical formula Na12Sr3Ca6Mn3Zr3WSi(Si9O27)2(Si3O9)2O(O,OH,H2O)3(OH,Cl)2. This formula is in extended form, to show the presence of cyclic silicate groups and domination of silicon at the M4 site, basing on the nomenclature of the eudialyte group. Some niobium substitutes for tungsten in khomyakovite. As suggested by its name, manganokhomyakovite is a manganese-analogue of khomyakovite, the latter being more rare. The two minerals are the only group representatives, beside taseqite, with species-defining strontium, although many other members display strontium diadochy. Manganokhomyakovite is the third eudialyte-group mineral with essential tungsten.
Oneillite is a rare mineral of the eudialyte group with the chemical formula Na15Ca3Mn3Fe2+3Zr3NbSiO(Si3O9)2(Si9O27)2(O,OH,H2O)3(OH,Cl)2. The formula is based on the original one but extended to show the presence of cyclic silicate groups and domination of Si at the M4 site. The mineral has lowered symmetry (space group R3, instead of more specific for the group R3m one) due to Ca-Mn ordering. Similar feature is displayed by some other eudialyte-group members: aqualite, labyrinthite, raslakite, and voronkovite. Oneillite is strongly enriched in rare earth elements (REE, mainly cerium), but REE do not dominate any of its sites.
Voronkovite is a very rare mineral of the eudialyte group with the chemical formula Na15(Na,Ca,Ce)3(Mn,Ca)3Fe3Zr3Si2Si24O72(OH,O)4Cl·H2O. The formula is based on the simplified original one; it does not show the presence of cyclic silicate groups, but two M3- and M4-site silicon atoms are shown separately (basing on the nomenclature of the eudialyte group). Voronkovite has lowered symmetry (space group R3, instead of more specific for the group R3m one), similarly to some other eudialyte-group members: aqualite, labyrinthite, oneillite and raslakite. The specific feature of voronkovite is, among others, strong enrichment in sodium.

Raslakite is a rare mineral of the eudialyte group with the chemical formula Na15Ca3Fe3(Na,Zr)3Zr3(Si,Nb)SiO(Si9O27)2(Si3O9)2(OH,H2O)3(Cl,OH). This formula is based on the original one, and is extended to show the presence of cyclic silicate groups. The additional silicon and oxygen shown in separation from the cyclic groups are in fact connected with two 9-fold rings. The mineral has lowered symmetry, similarly to some other eudialyte-group members: aqualite, labyrinthite, oneillite and voronkovite. The specific feature of raslakite is, among others, the presence of sodium and zirconium at the M2 site. Raslakite was named after Raslak Cirques located nearby the type locality.
References
- ↑ Warr, L.N. (2021). "IMA–CNMNC approved mineral symbols". Mineralogical Magazine. 85 (3): 291–320. Bibcode:2021MinM...85..291W. doi: 10.1180/mgm.2021.43 . S2CID 235729616.
- 1 2 3 4 5 Mindat, http://www.mindat.org/min-26453.html
- 1 2 3 4 5 Petersen, O.V., Johnsen, O., Gault, R.A., Niedermayr, G., and Grice, J.D., 2004. Taseqite, a new member of the eudialyte group from the Ilimaussaq alkaline complex, South Greenland. Neues Jahrbuch für Mineralogie Monatshefte Jg. 2004(2), 83–96
- ↑ Johnsen, O., Ferraris, G., Gault, R.A., Grice, D.G., Kampf, A.R., and Pekov, I.V., 2003. The nomenclature of eudialyte-group minerals. The Canadian Mineralogist 41, 785–794
- 1 2 Rastsvetaeva, R. K.; Chukanov, N. V.; Zaitsev, V. A.; Aksenov, S. M.; Viktorova, K. A. (May 2018). "Crystal Structure of Cl-Deficient Analogue of Taseqite from Odikhincha Massif". Crystallography Reports. 63 (3): 349–357. Bibcode:2018CryRp..63..349R. doi:10.1134/s1063774518030240. ISSN 1063-7745. S2CID 102659473.