Related Research Articles

Bradycardia, also called bradyarrhythmia, is a resting heart rate under 60 beats per minute (BPM). While bradycardia can result from various pathologic processes, it is commonly a physiologic response to cardiovascular conditioning or due to asymptomatic type 1 atrioventricular block.

The cardiac pacemaker is the heart's natural rhythm generator. It employs pacemaker cells that produce electrical impulses, known as cardiac action potentials, which control the rate of contraction of the cardiac muscle, that is, the heart rate. In most humans, these cells are concentrated in the sinoatrial (SA) node, the primary pacemaker, which regulates the heart’s sinus rhythm.

Sinus node dysfunction (SND), also known as sick sinus syndrome (SSS), is a group of abnormal heart rhythms (arrhythmias) usually caused by a malfunction of the sinus node, the heart's primary pacemaker. Tachycardia-bradycardia syndrome is a variant of sick sinus syndrome in which the arrhythmia alternates between fast and slow heart rates.

The sinoatrial node is an oval shaped region of special cardiac muscle in the upper back wall of the right atrium made up of cells known as pacemaker cells. The sinus node is approximately 15 mm long, 3 mm wide, and 1 mm thick, located directly below and to the side of the superior vena cava.
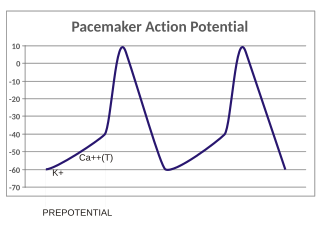
In the pacemaking cells of the heart (e.g., the sinoatrial node), the pacemaker potential (also called the pacemaker current) is the slow, positive increase in voltage across the cell's membrane (the membrane potential) that occurs between the end of one action potential and the beginning of the next action potential. This increase in membrane potential is what causes the cell membrane, which typically maintains a resting membrane potential around -65 mV, to reach the threshold potential and consequently fire the next action potential; thus, the pacemaker potential is what drives the self-generated rhythmic firing (automaticity) of pacemaker cells, and the rate of change (i.e., the slope) of the pacemaker potential is what determines the timing of the next action potential and thus the intrinsic firing rate of the cell. In a healthy sinoatrial node (SAN, a complex tissue within the right atrium containing pacemaker cells that normally determine the intrinsic firing rate for the entire heart), the pacemaker potential is the main determinant of the heart rate. Because the pacemaker potential represents the non-contracting time between heart beats (diastole), it is also called the diastolic depolarization. The amount of net inward current required to move the cell membrane potential during the pacemaker phase is extremely small, in the order of few pAs, but this net flux arises from time to time changing contribution of several currents that flow with different voltage and time dependence. Evidence in support of the active presence of K+, Ca2+, Na+ channels and Na+/K+ exchanger during the pacemaker phase have been variously reported in the literature, but several indications point to the “funny”(If) current as one of the most important.(see funny current). There is now substantial evidence that also sarcoplasmic reticulum (SR) Ca2+-transients participate to the generation of the diastolic depolarization via a process involving the Na–Ca exchanger.

Third-degree atrioventricular block is a medical condition in which the electrical impulse generated in the sinoatrial node in the atrium of the heart can not propagate to the ventricles.

The cardiac conduction system transmits the signals generated by the sinoatrial node – the heart's pacemaker, to cause the heart muscle to contract, and pump blood through the body's circulatory system. The pacemaking signal travels through the right atrium to the atrioventricular node, along the bundle of His, and through the bundle branches to Purkinje fibers in the walls of the ventricles. The Purkinje fibers transmit the signals more rapidly to stimulate contraction of the ventricles.
Heart block (HB) is a disorder in the heart's rhythm due to a fault in the natural pacemaker. This is caused by an obstruction – a block – in the electrical conduction system of the heart. Sometimes a disorder can be inherited. Despite the severe-sounding name, heart block may cause no symptoms at all in some cases, or occasional missed heartbeats in other cases, or may require the implantation of an artificial pacemaker, depending upon exactly where in the heart conduction is being impaired and how significantly it is affected.

Supraventricular tachycardia (SVT) is an umbrella term for fast heart rhythms arising from the upper part of the heart. This is in contrast to the other group of fast heart rhythms – ventricular tachycardia, which start within the lower chambers of the heart. There are four main types of SVT: atrial fibrillation, atrial flutter, paroxysmal supraventricular tachycardia (PSVT), and Wolff–Parkinson–White syndrome. The symptoms of SVT include palpitations, feeling of faintness, sweating, shortness of breath, and/or chest pain.

Multifocal atrial tachycardia (MAT) is an abnormal heart rhythm, specifically a type of supraventricular tachycardia, that is particularly common in older people and is associated with exacerbations of chronic obstructive pulmonary disease (COPD). Normally, the heart rate is controlled by a cluster of cells called the sinoatrial node. When a number of different clusters of cells outside the SA node take over control of the heart rate, and the rate exceeds 100 beats per minute, this is called multifocal atrial tachycardia.

Wandering atrial pacemaker (WAP) is an atrial rhythm where the pacemaking activity of the heart originates from different locations within the atria. This is different from normal pacemaking activity, where the sinoatrial node is responsible for each heartbeat and keeps a steady rate and rhythm. Causes of wandering atrial pacemaker are unclear, but there may be factors leading to its development. It is often seen in the young, the old, and in athletes, and rarely causes symptoms or requires treatment. Diagnosis of wandering atrial pacemaker is made by an ECG.

Junctional rhythm also called nodal rhythm describes an abnormal heart rhythm resulting from impulses coming from a locus of tissue in the area of the atrioventricular node, the "junction" between atria and ventricles.
A biological pacemaker is one or more types of cellular components that, when "implanted or injected into certain regions of the heart," produce specific electrical stimuli that mimic that of the body's natural pacemaker cells. Biological pacemakers are indicated for issues such as heart block, slow heart rate, and asynchronous heart ventricle contractions.

A sinoatrial block is a disorder in the normal rhythm of the heart, known as a heart block, that is initiated in the sinoatrial node. The initial action impulse in a heart is usually formed in the sinoatrial node and carried through the atria, down the internodal atrial pathways to the atrioventricular node (AV) node. In normal conduction, the impulse would travel across the bundle of His, down the bundle branches, and into the Purkinje fibers. This would depolarize the ventricles and cause them to contract.

An ectopic pacemaker, also known as ectopic focus or ectopic foci, is an excitable group of cells that causes a premature heart beat outside the normally functioning SA node of the heart. It is thus a cardiac pacemaker that is ectopic, producing an ectopic beat. Acute occurrence is usually non-life-threatening, but chronic occurrence can progress into tachycardia, bradycardia or ventricular fibrillation. In a normal heart beat rhythm, the SA node usually suppresses the ectopic pacemaker activity due to the higher impulse rate of the SA node. However, in the instance of either a malfunctioning SA node or an ectopic focus bearing an intrinsic rate superior to SA node rate, ectopic pacemaker activity may take over the natural heart rhythm. This phenomenon is called an escape rhythm, the lower rhythm having escaped from the dominance of the upper rhythm. As a rule, premature ectopic beats indicate increased myocyte or conducting tissue excitability, whereas late ectopic beats indicate proximal pacemaker or conduction failure with an escape 'ectopic' beat.
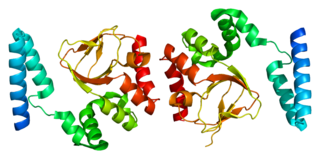
Potassium/sodium hyperpolarization-activated cyclic nucleotide-gated channel 4 is a protein that in humans is encoded by the HCN4 gene.

Ankyrin-2, also known as Ankyrin-B, and Brain ankyrin, is a protein which in humans is encoded by the ANK2 gene. Ankyrin-2 is ubiquitously expressed, but shows high expression in cardiac muscle. Ankyrin-2 plays an essential role in the localization and membrane stabilization of ion transporters and ion channels in cardiomyocytes, as well as in costamere structures. Mutations in ANK2 cause a dominantly-inherited, cardiac arrhythmia syndrome known as long QT syndrome 4 as well as sick sinus syndrome; mutations have also been associated to a lesser degree with hypertrophic cardiomyopathy. Alterations in ankyrin-2 expression levels are observed in human heart failure.

Arrhythmias, also known as cardiac arrhythmias, are irregularities in the heartbeat, including when it is too fast or too slow. A resting heart rate that is too fast – above 100 beats per minute in adults – is called tachycardia, and a resting heart rate that is too slow – below 60 beats per minute – is called bradycardia. Some types of arrhythmias have no symptoms. Symptoms, when present, may include palpitations or feeling a pause between heartbeats. In more serious cases, there may be lightheadedness, passing out, shortness of breath, chest pain, or decreased level of consciousness. While most cases of arrhythmia are not serious, some predispose a person to complications such as stroke or heart failure. Others may result in sudden death.
Cardiac physiology or heart function is the study of healthy, unimpaired function of the heart: involving blood flow; myocardium structure; the electrical conduction system of the heart; the cardiac cycle and cardiac output and how these interact and depend on one another.

BRL-32872 is an experimental drug candidate that provides a novel approach to the treatment of cardiac arrhythmia. Being a derivative of verapamil, it possesses the ability to inhibit Ca+2 membrane channels. Specific modifications in hydrogen bonding activity, nitrogen lone pair availability, and molecular flexibility allow BRL-32872 to inhibit K+ channels as well. As such, BRL-32872 is classified as both a class III (K+ blocking) and class IV (Ca+2 blocking) antiarrhythmic agent.
References
- ↑ Kapoor, N., Liang, W., Marbán, E., and Cheol Cho, H. (2013). Direct conversion of quiescent cardiomyocytes to pacemaker cells by expression of Tbx18. Nature Biotechnology. 31: 54-62.
- ↑ Kapoor, N., Liang, W., Marbán, E., and Cheol Cho, H. (2013). Direct conversion of quiescent cardiomyocytes to pacemaker cells by expression of Tbx18. Nature Biotechnology. 31: 54-62.
- ↑ Tung, R., Shen, W., Hayes, D., Hammill, S., Bailey, K., and Gersh, B. (1994). Long-Term Survival After Permanent Pacemaker Implantation for Sick Sinus Syndrome. The American Journal of Cardiology. 74: 1016–1020.
- ↑ Wiese, C., Grieskamp, T., Airik, R., Mommersteeg, M., Gardiwal, A., deVries, C., Gossler, K., Moorman, A., Kispert, A., and Christoffels, V. (2009). Formation of the Sinus Node Head and Differentiation of Sinus Node Myocardium Are Independently Regulated by Tbx18 and Tbx3. Circulation Research. 104: 388-397.
- ↑ Kapoor, N., Liang, W., Marbán, E., and Cheol Cho, H. (2013). Direct conversion of quiescent cardiomyocytes to pacemaker cells by expression of Tbx18. Nature Biotechnology. 31: 54-62.
- ↑ Y-F. Hu, J. F. Dawkins, H. C. Cho, E. Marbán, E. Cingolani,(2014).Biological pacemaker created by minimally invasive somatic reprogramming in pigs with complete heart block. Sci. Transl. Med. 6, 245ra94
- ↑ Li, R.A. (2012). Gene-and cell-based bio-artificial pacemaker: what basic and translational lessons have we learned? Gene Therapy. 19: 588-595.