Bradycardia, also called bradyarrhythmia, is a resting heart rate under 60 beats per minute (BPM). While bradycardia can result from various pathologic processes, it is commonly a physiologic response to cardiovascular conditioning or due to asymptomatic type 1 atrioventricular block.

The heart is a muscular organ found in most animals. This organ pumps blood through the blood vessels. Heart and blood vessels together make the circulatory system. The pumped blood carries oxygen and nutrients to the tissue, while carrying metabolic waste such as carbon dioxide to the lungs. In humans, the heart is approximately the size of a closed fist and is located between the lungs, in the middle compartment of the chest, called the mediastinum.

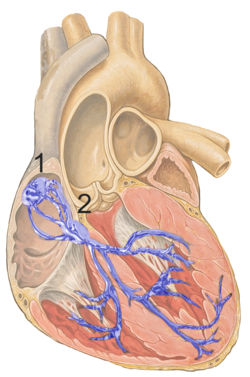
The cardiac pacemaker is the heart's natural rhythm generator. It employs pacemaker cells that produce electrical impulses, known as cardiac action potentials, which control the rate of contraction of the cardiac muscle, that is, the heart rate. In most humans, these cells are concentrated in the sinoatrial (SA) node, the primary pacemaker, which regulates the heart’s sinus rhythm.

Systole is the part of the cardiac cycle during which some chambers of the heart contract after refilling with blood. Its contrasting phase is diastole, the relaxed phase of the cardiac cycle when the chambers of the heart are refilling with blood.

Sinus node dysfunction (SND), also known as sick sinus syndrome (SSS), is a group of abnormal heart rhythms (arrhythmias) usually caused by a malfunction of the sinus node, the heart's primary pacemaker. Tachycardia-bradycardia syndrome is a variant of sick sinus syndrome in which the arrhythmia alternates between fast and slow heart rates.

Cardiac muscle is one of three types of vertebrate muscle tissues, the others being skeletal muscle and smooth muscle. It is an involuntary, striated muscle that constitutes the main tissue of the wall of the heart. The cardiac muscle (myocardium) forms a thick middle layer between the outer layer of the heart wall and the inner layer, with blood supplied via the coronary circulation. It is composed of individual cardiac muscle cells joined by intercalated discs, and encased by collagen fibers and other substances that form the extracellular matrix.
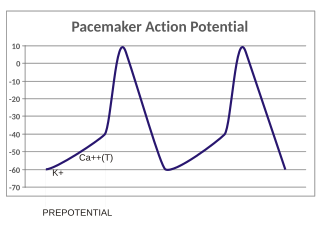
In the pacemaking cells of the heart (e.g., the sinoatrial node), the pacemaker potential (also called the pacemaker current) is the slow, positive increase in voltage across the cell's membrane (the membrane potential) that occurs between the end of one action potential and the beginning of the next action potential. This increase in membrane potential is what causes the cell membrane, which typically maintains a resting membrane potential around -65 mV, to reach the threshold potential and consequently fire the next action potential; thus, the pacemaker potential is what drives the self-generated rhythmic firing (automaticity) of pacemaker cells, and the rate of change (i.e., the slope) of the pacemaker potential is what determines the timing of the next action potential and thus the intrinsic firing rate of the cell. In a healthy sinoatrial node (SAN, a complex tissue within the right atrium containing pacemaker cells that normally determine the intrinsic firing rate for the entire heart), the pacemaker potential is the main determinant of the heart rate. Because the pacemaker potential represents the non-contracting time between heart beats (diastole), it is also called the diastolic depolarization. The amount of net inward current required to move the cell membrane potential during the pacemaker phase is extremely small, in the order of few pAs, but this net flux arises from time to time changing contribution of several currents that flow with different voltage and time dependence. Evidence in support of the active presence of K+, Ca2+, Na+ channels and Na+/K+ exchanger during the pacemaker phase have been variously reported in the literature, but several indications point to the “funny”(If) current as one of the most important.(see funny current). There is now substantial evidence that also sarcoplasmic reticulum (SR) Ca2+-transients participate to the generation of the diastolic depolarization via a process involving the Na–Ca exchanger.

The cardiac conduction system transmits the signals generated by the sinoatrial node – the heart's pacemaker, to cause the heart muscle to contract, and pump blood through the body's circulatory system. The pacemaking signal travels through the right atrium to the atrioventricular node, along the bundle of His, and through the bundle branches to Purkinje fibers in the walls of the ventricles. The Purkinje fibers transmit the signals more rapidly to stimulate contraction of the ventricles.

Unlike the action potential in skeletal muscle cells, the cardiac action potential is not initiated by nervous activity. Instead, it arises from a group of specialized cells known as pacemaker cells, that have automatic action potential generation capability. In healthy hearts, these cells form the cardiac pacemaker and are found in the sinoatrial node in the right atrium. They produce roughly 60–100 action potentials every minute. The action potential passes along the cell membrane causing the cell to contract, therefore the activity of the sinoatrial node results in a resting heart rate of roughly 60–100 beats per minute. All cardiac muscle cells are electrically linked to one another, by intercalated discs which allow the action potential to pass from one cell to the next. This means that all atrial cells can contract together, and then all ventricular cells.
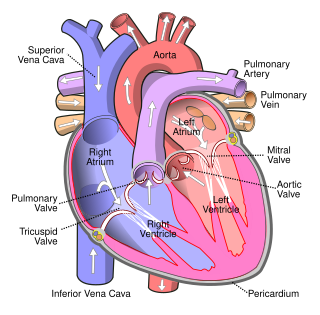
The atrium is one of the two upper chambers in the heart that receives blood from the circulatory system. The blood in the atria is pumped into the heart ventricles through the atrioventricular mitral and tricuspid heart valves.

The cardiac cycle is the performance of the human heart from the beginning of one heartbeat to the beginning of the next. It consists of two periods: one during which the heart muscle relaxes and refills with blood, called diastole, following a period of robust contraction and pumping of blood, called systole. After emptying, the heart relaxes and expands to receive another influx of blood returning from the lungs and other systems of the body, before again contracting to pump blood to the lungs and those systems.

In the heart's conduction system, Bachmann's bundle is a branch of the anterior internodal tract that resides on the inner wall of the left atrium. It is a broad band of cardiac muscle that passes from the right atrium, between the superior vena cava and the ascending aorta. Bachmann's bundle is, during normal sinus rhythm, the preferential path for electrical activation of the left atrium. It is therefore considered to be part of the "atrial conduction system" of the heart.

The sinoatrial nodal artery, sinoatrial nodal artery or sinoatrial artery is an artery of the heart which supplies the sinoatrial node, the natural pacemaker center of the heart. It is usually a branch of the right coronary artery. It passes between the right atrium, and the opening of the superior vena cava.
The pacemaker current is an electric current in the heart that flows through the HCN channel or pacemaker channel. Such channels are important parts of the electrical conduction system of the heart and form a component of the natural pacemaker.
Sinoatrial arrest is a medical condition wherein the sinoatrial node of the heart transiently ceases to generate the electrical impulses that normally stimulate the myocardial tissues to contract and thus the heart to beat. It is defined as lasting from 2.0 seconds to several minutes. Since the heart contains multiple pacemakers, this interruption of the cardiac cycle generally lasts only a few seconds before another part of the heart, such as the atrio-ventricular junction or the ventricles, begins pacing and restores the heart action. This condition can be detected on an electrocardiogram (ECG) as a brief period of irregular length with no electrical activity before either the sinoatrial node resumes normal pacing, or another pacemaker begins pacing. If a pacemaker other than the sinoatrial node is pacing the heart, this condition is known as an escape rhythm. If no other pacemaker begins pacing during an episode of sinus arrest it becomes a cardiac arrest. This condition is sometimes confused with sinoatrial block, a condition in which the pacing impulse is generated, but fails to conduct through the myocardium. Differential diagnosis of the two conditions is possible by examining the exact length of the interruption of cardiac activity. If the next available pacemaker takes over, it is in the following order:

An ectopic pacemaker, also known as ectopic focus or ectopic foci, is an excitable group of cells that causes a premature heart beat outside the normally functioning SA node of the heart. It is thus a cardiac pacemaker that is ectopic, producing an ectopic beat. Acute occurrence is usually non-life-threatening, but chronic occurrence can progress into tachycardia, bradycardia or ventricular fibrillation. In a normal heart beat rhythm, the SA node usually suppresses the ectopic pacemaker activity due to the higher impulse rate of the SA node. However, in the instance of either a malfunctioning SA node or an ectopic focus bearing an intrinsic rate superior to SA node rate, ectopic pacemaker activity may take over the natural heart rhythm. This phenomenon is called an escape rhythm, the lower rhythm having escaped from the dominance of the upper rhythm. As a rule, premature ectopic beats indicate increased myocyte or conducting tissue excitability, whereas late ectopic beats indicate proximal pacemaker or conduction failure with an escape 'ectopic' beat.
Hyperpolarization-activated cyclic nucleotide–gated (HCN) channels are integral membrane proteins that serve as nonselective voltage-gated cation channels in the plasma membranes of heart and brain cells. HCN channels are sometimes referred to as pacemaker channels because they help to generate rhythmic activity within groups of heart and brain cells. HCN channels are activated by membrane hyperpolarization, are permeable to Na + and K +, and are constitutively open at voltages near the resting membrane potential. HCN channels are encoded by four genes and are widely expressed throughout the heart and the central nervous system.
Cardiac physiology or heart function is the study of healthy, unimpaired function of the heart: involving blood flow; myocardium structure; the electrical conduction system of the heart; the cardiac cycle and cardiac output and how these interact and depend on one another.

Heart development, also known as cardiogenesis, refers to the prenatal development of the heart. This begins with the formation of two endocardial tubes which merge to form the tubular heart, also called the primitive heart tube. The heart is the first functional organ in vertebrate embryos.
Cardiac excitation-contraction coupling (CardiacEC coupling) describes the series of events, from the production of an electrical impulse (action potential) to the contraction of muscles in the heart. This process is of vital importance as it allows for the heart to beat in a controlled manner, without the need for conscious input. EC coupling results in the sequential contraction of the heart muscles that allows blood to be pumped, first to the lungs (pulmonary circulation) and then around the rest of the body (systemic circulation) at a rate between 60 and 100 beats every minute, when the body is at rest. This rate can be altered, however, by nerves that work to either increase heart rate (sympathetic nerves) or decrease it (parasympathetic nerves), as the body's oxygen demands change. Ultimately, muscle contraction revolves around a charged atom (ion), calcium (Ca2+), which is responsible for converting the electrical energy of the action potential into mechanical energy (contraction) of the muscle. This is achieved in a region of the muscle cell, called the transverse tubule during a process known as calcium induced calcium release.