Terpene alcohol may refer to a variety of terpenoids, i.e. terpenes modified with one or more hydroxy groups:
Terpenols
Geraniol, nerol, linalool, and citronellol constitute the rose alcohols
Sesquiterpenols
Tetraterpenes
Terpene alcohol may refer to a variety of terpenoids, i.e. terpenes modified with one or more hydroxy groups:
Terpenols
Geraniol, nerol, linalool, and citronellol constitute the rose alcohols
Sesquiterpenols
Tetraterpenes

Rose water is a flavoured water made by steeping rose petals in water. It is the hydrosol portion of the distillate of rose petals, a by-product of the production of rose oil for use in perfume. Rose water is also used to flavour food, as a component in some cosmetic and medical preparations, and for religious purposes throughout Eurasia.

Citronella oil is an essential oil obtained from the leaves and stems of different species of Cymbopogon (lemongrass). The oil is used extensively as a source of perfumery chemicals such as citronellal, citronellol, and geraniol. These chemicals find extensive use in soap, candles and incense, perfumery, cosmetic, and flavouring industries throughout the world.

Geraniol is a monoterpenoid and an alcohol. It is the primary component of citronella oil and is a primary component of rose oil and palmarosa oil. It is a colorless oil, although commercial samples can appear yellow. It has low solubility in water, but it is soluble in common organic solvents. The functional group derived from geraniol is called geranyl.

Rhodinol is the chemical compound 3,7-dimethyloct-7-en-1-ol. As the (3S) isomer it is CAS 6812-78-8, and as the racemate it is CAS 141-25-3.

Linalool refers to two enantiomers of a naturally occurring terpene alcohol found in many flowers and spice plants. Linalool has multiple commercial applications, the majority of which are based on its pleasant scent. A colorless oil, linalool is classified as an acyclic monoterpenoid. In plants, it is a metabolite, a volatile oil component, an antimicrobial agent, and an aroma compound. Linalool has uses in manufacturing of soaps, fragrances, food additives as flavors, household products, and insecticides. Esters of linalool are referred to as linalyl, e.g. linalyl pyrophosphate, an isomer of geranyl pyrophosphate.

Rose oil is the essential oil extracted from the petals of various types of rose. Rose ottos are extracted through steam distillation, while rose absolutes are obtained through solvent extraction, the absolute being used more commonly in perfumery. The production technique originated in Greater Iran. Even with their high price and the advent of organic synthesis, rose oils are still perhaps the most widely used essential oil in perfumery.

Neroli oil is an essential oil produced from the blossom of the bitter orange tree. Its scent is sweet, honeyed and somewhat metallic with green and spicy facets. Orange blossom is also extracted from the same blossom and both extracts are extensively used in perfumery. Orange blossom can be described as smelling sweeter, warmer and more floral than neroli. The difference between how neroli and orange blossom smell and why they are referred to with different names, is a result of the process of extraction that is used to obtain the oil from the blooms. Neroli is extracted by steam distillation and orange blossom is extracted via a process of enfleurage or solvent extraction.

Laundry detergent is a type of detergent used for cleaning dirty laundry (clothes). Laundry detergent is manufactured in powder and liquid form.

Myrcene, or β-myrcene, is a monoterpene. A colorless oil, it occurs widely in essential oils. It is produced mainly semi-synthetically from Myrcia, from which it gets its name. It is an intermediate in the production of several fragrances. α-Myrcene is the name for the isomer 2-methyl-6-methylene-1,7-octadiene, which has not been found in nature.

Citronellol, or dihydrogeraniol, is a natural acyclic monoterpenoid. Both enantiomers occur in nature. (+)-Citronellol, which is found in citronella oils, including Cymbopogon nardus (50%), is the more common isomer. (−)-Citronellol is widespread, but particularly abundant in the oils of rose (18–55%) and Pelargonium geraniums.

Nerol is a monoterpenoid alcohol found in many essential oils such as lemongrass and hops. It was originally isolated from neroli oil, hence its name. This colourless liquid is used in perfumery. Like geraniol, nerol has a sweet rose odor but it is considered to be fresher. Esters and related derivatives of nerol are referred to as neryl, e.g., neryl acetate.

Geranyl pyrophosphate (GPP), also known as geranyl diphosphate (GDP), is the pyrophosphate ester of the terpenoid geraniol. Its salts are colorless. It is a precursor to many natural products.
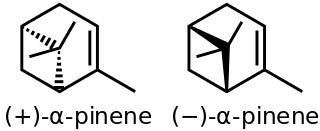
α-Pinene is an organic compound of the terpene class. It is one of the two isomers of pinene, the other being β-pinene. An alkene, it contains a reactive four-membered ring. It is found in the oils of many species of coniferous trees, notably Pinus and Picea species. It is also found in the essential oil of rosemary and Satureja myrtifolia. Both enantiomers are known in nature; (1S,5S)- or (−)-α-pinene is more common in European pines, whereas the (1R,5R)- or (+)-α-isomer is more common in North America. The enantiomers' racemic mixture is present in some oils such as eucalyptus oil and orange peel oil.
Monoterpenes are a class of terpenes that consist of two isoprene units and have the molecular formula C10H16. Monoterpenes may be linear (acyclic) or contain rings (monocyclic and bicyclic). Modified terpenes, such as those containing oxygen functionality or missing a methyl group, are called monoterpenoids. Monoterpenes and monoterpenoids are diverse. They have relevance to the pharmaceutical, cosmetic, agricultural, and food industries.

Cymbopogon citratus, commonly known as West Indian lemon grass or simply lemon grass, is a tropical plant native to South Asia and Maritime Southeast Asia and introduced to many tropical regions.

The aromas of wine are more diverse than its flavours. The human tongue is limited to the primary tastes perceived by taste receptors on the tongue – sourness, bitterness, saltiness, sweetness and savouriness. The wide array of fruit, earthy, leathery, floral, herbal, mineral, and woodsy flavour present in wine are derived from aroma notes sensed by the olfactory bulb. In wine tasting, wine is sometimes smelled before taking a sip in order to identify some components of the wine that may be present. Different terms are used to describe what is being smelled. The most basic term is aroma which generally refers to a "pleasant" smell as opposed to odour which refers to an unpleasant smell or possible wine fault. The term aroma may be further distinguished from bouquet which generally refers to the smells that arise from the chemical reactions of fermentation and aging of the wine.
The molecular formula C10H18O (molar mass : 154.25 g/mol) may refer to:

Wine is a complex mixture of chemical compounds in a hydro-alcoholic solution with a pH around 4. The chemistry of wine and its resultant quality depend on achieving a balance between three aspects of the berries used to make the wine: their sugar content, acidity and the presence of secondary compounds. Vines store sugar in grapes through photosynthesis, and acids break down as grapes ripen. Secondary compounds are also stored in the course of the season. Anthocyanins give grapes a red color and protection against ultraviolet light. Tannins add bitterness and astringency which acts to defend vines against pests and grazing animals.
Linalool dehydratase (EC 4.2.1.127, linalool hydro-lyase (myrcene-forming)) is an enzyme with systematic name (3S)-linalool hydro-lyase (myrcene-forming). This enzyme catalyses the following chemical reaction

Helichrysum stoechas, known as Mediterranean strawflower, curry plant, common shrubby everlasting, everlasting flower, or eternal flower, is an annual or perennial shrub that prefers dry, rocky and sandy areas. It can grow up to 120 centimeters in height, and spreads over 1 square meter in area. It is a hermaphrodite that has grayish green leaves and produces small globular yellow flowers sometimes in the Spring or in July and August that are pollinated by insects.