A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei, and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive elements where both electronic and nuclear changes can occur.

Crotonic acid ((2E)-but-2-enoic acid) or is a short-chain unsaturated carboxylic acid, described by the formula CH3CH=CHCO2H. It is called crotonic acid because it was erroneously thought to be a saponification product of croton oil. It crystallizes as colorless needles from hot water. The cis-isomer of crotonic acid is called isocrotonic acid. Crotonic acid is soluble in water and many organic solvents. Its odor is similar to butyric acid.
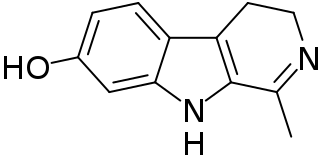
Harmalol is a bioactive beta-carboline and a member of the harmala alkaloids.
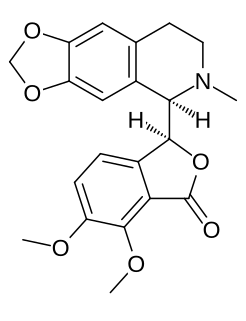
Hydrastine is an alkaloid which was discovered in 1851 by Alfred P. Durand. Hydrolysis of hydrastine yields hydrastinine, which was patented by Bayer as a haemostatic drug during the 1910s. It is present in Hydrastis canadensis and other plants of the family Ranunculaceae.

Thiobutabarbital is a short-acting barbiturate derivative invented in the 1950s. It has sedative, anticonvulsant and hypnotic effects, and is still used in veterinary medicine for induction in surgical anaesthesia
Alkyl sulfonates are esters of alkane sulfonic acids with the general formula R-SO2-O-R'. They act as alkylating agents, some of them are used as alkylating antineoplastic agents in the treatment of cancer, e.g. Busulfan.
Tropaeolin is the retained name for some azo dyes from the industrially applied group of acid dyes used as pH indicators. The name is derived from the botanical name Tropaeolum for nasturtiums.

Pitofenone is an antispasmodic.

Norleucine (abbreviated as Nle) is an amino acid with the formula CH3(CH2)3CH(NH2)CO2H. A systematic name for this compound is 2-aminohexanoic acid. The compound is an isomer of the more common amino acid leucine. Like most other α-amino acids, norleucine is chiral. It is a white, water-soluble solid.

Protoanemonin is a toxin found in all plants of the buttercup family (Ranunculaceae). When the plant is wounded or macerated, the unstable glucoside found in the plant, ranunculin, is enzymatically broken down into glucose and the toxic protoanemonin. It is the lactone of 4-hydroxy-2,4-pentadienoic acid.

Anthraquinone dyes are an abundant group of dyes comprising a anthraquinone unit as the shared structural element. Anthraquinone itself is colourless, but red to blue dyes are obtained by introducing electron donor groups such as hydroxy or amino groups in the 1-, 4-, 5- or 8-position. Anthraquinone dyestuffs are structurally related to indigo dyestuffs and are classified together with these in the group of carbonyl dyes.
Methine dyes are dyes whose chromophoric system consists of conjugated double bonds (polyenes) flanked by two end groups: an electron acceptor A and an electron donor D.
Structural of methine dyes
Penicillium jensenii is an anamorph species of the genus of Penicillium which produces citrinin, griseofulvin and fumagillin.
Penicillium melinii is an anamorph species of the genus Penicillium which produces griseofulvin and beta-Nitropropionic acid.
Penicillium rubrum is a species of fungus in the genus Penicillium which produces kojic acid, mitorubrin, mitorubrinol, rubratoxin A, rubratoxin B rubralactone, rubramin and occurs in grain corn and soybeans. Penicillium rubrum is similar to the species Penicillium chrysogenum.
Penicillium tardum is an anamorph species of fungus in the genus Penicillium which produces rugulosin.
A descriptor is in chemical nomenclature a prefix placed before the systematic substance name, which describes the configuration or the stereochemistry of the molecule. Some listed descriptors are only of historical interest and should not be used in publications anymore as they do not correspond with the modern recommendations of the IUPAC. Stereodescriptors are often used in combination with locants to clearly identify a chemical structure unambiguously.

Blackplate is hot rolled or cold rolled, non-descaled sheet steel or sheet iron.

2,4,6-Trimethylpyridine (2,4,6-collidine) is an organic compound which belongs to the heterocycles. It consists of a pyridine ring substituted with three methyl groups. It belongs to the substance group of the collidines, a group of six constitutional isomers. 2,4,6-trimethylpyridine is the most well-known isomer of this group.

Polycarboxylates are linear polymers with a high molecular mass and with many carboxylate groups. They are polymers of acrylic acid or copolymers of acrylic acid and maleic acid. The polymer is used as the sodium salt.