
Mupirocin, sold under the brand name Bactroban among others, is a topical antibiotic useful against superficial skin infections such as impetigo or folliculitis. It may also be used to get rid of methicillin-resistant S. aureus (MRSA) when present in the nose without symptoms. Due to concerns of developing resistance, use for greater than ten days is not recommended. It is used as a cream or ointment applied to the skin.

Geldanamycin is a 1,4-benzoquinone ansamycin antitumor antibiotic that inhibits the function of Hsp90 by binding to the unusual ADP/ATP-binding pocket of the protein. HSP90 client proteins play important roles in the regulation of the cell cycle, cell growth, cell survival, apoptosis, angiogenesis and oncogenesis.
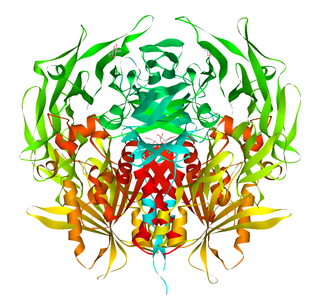
Dipeptidyl peptidase-4, also known as adenosine deaminase complexing protein 2 or CD26 is a protein that, in humans, is encoded by the DPP4 gene. DPP4 is related to FAP, DPP8, and DPP9. The enzyme was discovered in 1966 by Hopsu-Havu and Glenner, and as a result of various studies on chemism, was called dipeptidyl peptidase IV [DP IV].
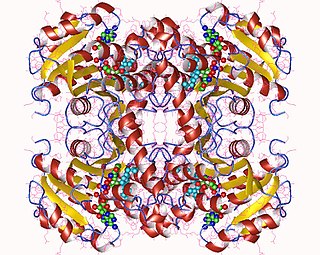
Enoyl-acyl carrier protein reductase, is a key enzyme of the type II fatty acid synthesis (FAS) system. ENR is an attractive target for narrow-spectrum antibacterial drug discovery because of its essential role in metabolism and its sequence conservation across many bacterial species. In addition, the bacterial ENR sequence and structural organization are distinctly different from those of mammalian fatty acid biosynthesis enzymes.

Prolyl endopeptidase (PE) also known as prolyl oligopeptidase or post-proline cleaving enzyme is an enzyme that in humans is encoded by the PREP gene.

Procollagen-proline dioxygenase, commonly known as prolyl hydroxylase, is a member of the class of enzymes known as alpha-ketoglutarate-dependent hydroxylases. These enzymes catalyze the incorporation of oxygen into organic substrates through a mechanism that requires alpha-Ketoglutaric acid, Fe2+, and ascorbate. This particular enzyme catalyzes the formation of (2S, 4R)-4-hydroxyproline, a compound that represents the most prevalent post-translational modification in the human proteome.

Fibroblast activation protein alpha (FAP-alpha) also known as prolyl endopeptidase FAP is an enzyme that in humans is encoded by the FAP gene.

Zinc finger and BTB domain-containing protein 32 is a protein that in humans is encoded by the 1960 bp ZBTB32 gene. The 52 kDa protein is a transcriptional repressor and the gene is expressed in T and B cells upon activation, but also significantly in testis cells. It is a member of the Poxviruses and Zinc-finger (POZ) and Krüppel (POK) family of proteins, and was identified in multiple screens involving either immune cell tumorigenesis or immune cell development.

Polyozellus multiplex is a species complex of fungi first described in 1899. P. multiplex is commonly known as the blue chanterelle, the purple chanterelle, or, in Alaska, the black chanterelle. However, this mushroom is not closely related to true chanterelles. While this name used to refer to a group of species, it is now used to describe only one species that held onto the name P. multiplex. The fruiting bodies of this species are blue- to purple-colored clusters of vase- or spoon-shaped caps, with veiny wrinkles on the underside which run down the length of the stem. P. multiplex was considered the monotypic species of the genus Polyozellus until recent molecular research divided the P. multiplex species complex into five species. The genus name is derived from the Greek poly meaning many, and oz, meaning branch. The specific epithet multiplex means "in many pieces," referring to the compound nature of the fruiting body.
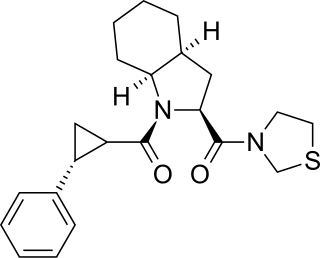
S-17092 is a drug which acts as a selective inhibitor of the enzyme prolyl endopeptidase. This enzyme is involved in the metabolic breakdown of a number of neuropeptide neurotransmitters in the brain, and so inhibiting the action of the enzyme increases the activity of these neuropeptides. This produces nootropic effects which make S-17092 a promising and novel treatment for neurodegenerative conditions such as Alzheimer's disease and Parkinson's disease.

Kynapcin is a general name for a number of dibenzofuranyl derivatives of the molecule polyozellin, present in the fungus Polyozellus multiplex. Like polyozellin, some kynapcins inhibit prolyl endopeptidase, an enzyme that has a role in processing proteins including amyloid precursor protein. Chemicals that inhibit prolyl endopeptidase have attracted research interest due to their potential therapeutic effects. Several kynapcins have been found in P. multiplex, each with different chemical properties, including kynapcin-12, kynapcin-13 and -28, and -24. A total synthesis of kynapcin-24 was achieved in 2009.

Polyozellin is a chemical which occurs in the mushroom Polyozellus multiplex. It inhibits prolyl endopeptidase, an enzyme that has a role in processing proteins in Alzheimer's disease. Chemicals that inhibit prolyl endopeptidase have attracted research interest due to their potential therapeutic effects. Structurally related dibenzofuranyl derivatives of polyozellin are known as kynapcins.

An Oligopeptidase is an enzyme that cleaves peptides but not proteins. This property is due to its structure: the active site of this enzyme is located at the end of a narrow cavity which can only be reached by peptides.
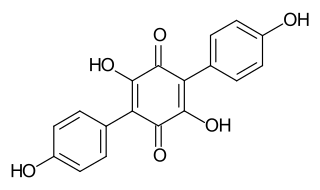
Atromentin is a natural chemical compound found in Agaricomycetes fungi in the orders Agaricales and Thelephorales. It can also be prepared by laboratory synthesis. Chemically, it is a polyphenol and a benzoquinone.

Omphalotus subilludens, commonly known as the Southern Jack O'lantern mushroom, is a basidiomycete fungi in the genus Omphalotus. It has been definitively recorded in Florida and Texas with reports of species in Arizona and Mexico. It fruits on dead and dying trees during warmer parts of the year, producing a fairly large orange to brown-orange fruiting body that occurs in clusters. It is most closely related to O. olivascans, O. olearius, and O. japonicus and has high cross compatibility with O. olivescans and O. olearis. It is poisonous to humans and animals when eaten but rarely produces life-threatening symptoms, usually poisonings are resolved in 24-48 hours, with the majority of symptoms being gastrointestinal. Compounds in these mushrooms have pharmacological potential with potential applications in anti-coagulants, cancer therapies, and antibiotics. It is also bioluminescent producing a faint glow around the gills through the oxidation of luciferase.

Gintonin is a glycolipoprotein fraction isolated from Panax ginseng. The non-saponin ingredient was designated as gintonin, where gin was derived from ginseng, ton from the tonic effects of ginseng, and in from protein. The main component of gintonin is a complex of lysophosphatidic acids (LPA) and ginseng proteins such as ginseng major latex-like protein151 (GLP151) and ginseng ribonuclease-like storage protein.
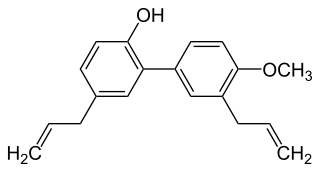
4-O-Methylhonokiol is a neolignan, a type of phenolic compound. It is found in the bark of Magnolia grandiflora and in M. virginiana flowers.

Glutamyl endopeptidase is an extracellular bacterial serine protease of the glutamyl endopeptidase I family that was initially isolated from the Staphylococcus aureus strain V8. The protease is, hence, commonly referred to as "V8 protease", or alternatively SspA from its corresponding gene.
Fungal isolates have been researched for decades. Because fungi often exist in thin mycelial monolayers, with no protective shell, immune system, and limited mobility, they have developed the ability to synthesize a variety of unusual compounds for survival. Researchers have discovered fungal isolates with anticancer, antimicrobial, immunomodulatory, and other bio-active properties. The first statins, β-Lactam antibiotics, as well as a few important antifungals, were discovered in fungi.
Christopher Joseph Schofield is a Professor of Chemistry at the University of Oxford and a Fellow of the Royal Society. Chris Schofield is a professor of organic chemistry at the University of Oxford, Department of Chemistry and a Fellow of Hertford College. Schofield studied functional, structural and mechanistic understanding of enzymes that employ oxygen and 2-oxoglutarate as a co-substrate. His work has opened up new possibilities in antibiotic research, oxygen sensing, and gene regulation.



















