Related Research Articles

A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis.

In organic chemistry, benzyl is the substituent or molecular fragment possessing the structure R−CH2−C6H5. Benzyl features a benzene ring attached to a methylene group group.

In organic chemistry, peptide synthesis is the production of peptides, compounds where multiple amino acids are linked via amide bonds, also known as peptide bonds. Peptides are chemically synthesized by the condensation reaction of the carboxyl group of one amino acid to the amino group of another. Protecting group strategies are usually necessary to prevent undesirable side reactions with the various amino acid side chains. Chemical peptide synthesis most commonly starts at the carboxyl end of the peptide (C-terminus), and proceeds toward the amino-terminus (N-terminus). Protein biosynthesis in living organisms occurs in the opposite direction.

The Nicolaou Taxol total synthesis, published by K. C. Nicolaou and his group in 1994 concerns the total synthesis of taxol. Taxol is an important drug in the treatment of cancer but also expensive because the compound is harvested from a scarce resource, namely the pacific yew.
Silyl ethers are a group of chemical compounds which contain a silicon atom covalently bonded to an alkoxy group. The general structure is R1R2R3Si−O−R4 where R4 is an alkyl group or an aryl group. Silyl ethers are usually used as protecting groups for alcohols in organic synthesis. Since R1R2R3 can be combinations of differing groups which can be varied in order to provide a number of silyl ethers, this group of chemical compounds provides a wide spectrum of selectivity for protecting group chemistry. Common silyl ethers are: trimethylsilyl (TMS), tert-butyldiphenylsilyl (TBDPS), tert-butyldimethylsilyl (TBS/TBDMS) and triisopropylsilyl (TIPS). They are particularly useful because they can be installed and removed very selectively under mild conditions.
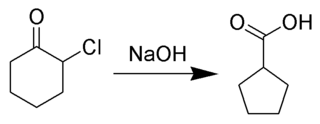
The Favorskii rearrangement is principally a rearrangement of cyclopropanones and α-halo ketones that leads to carboxylic acid derivatives. In the case of cyclic α-halo ketones, the Favorskii rearrangement constitutes a ring contraction. This rearrangement takes place in the presence of a base, sometimes hydroxide, to yield a carboxylic acid but most of the time either an alkoxide base or an amine to yield an ester or an amide, respectively. α,α'-Dihaloketones eliminate HX under the reaction conditions to give α,β-unsaturated carbonyl compounds.

The Danishefsky Taxol total synthesis in organic chemistry is an important third Taxol synthesis published by the group of Samuel Danishefsky in 1996 two years after the first two efforts described in the Holton Taxol total synthesis and the Nicolaou Taxol total synthesis. Combined they provide a good insight in the application of organic chemistry in total synthesis.

The Holton Taxol total synthesis, published by Robert A. Holton and his group at Florida State University in 1994, was the first total synthesis of Taxol.

The Dakin oxidation is an organic redox reaction in which an ortho- or para-hydroxylated phenyl aldehyde or ketone reacts with hydrogen peroxide in base to form a benzenediol and a carboxylate. Overall, the carbonyl group is oxidized, and the hydrogen peroxide is reduced.

2-Mercaptoethanol (also β-mercaptoethanol, BME, 2BME, 2-ME or β-met) is the chemical compound with the formula HOCH2CH2SH. ME or βME, as it is commonly abbreviated, is used to reduce disulfide bonds and can act as a biological antioxidant by scavenging hydroxyl radicals (amongst others). It is widely used because the hydroxyl group confers solubility in water and lowers the volatility. Due to its diminished vapor pressure, its odor, while unpleasant, is less objectionable than related thiols.
Pivalic acid is a carboxylic acid with a molecular formula of (CH3)3CCO2H. This colourless, odiferous organic compound is solid at room temperature. A common abbreviation for the pivalyl or pivaloyl group (t-BuC(O)) is Piv and for pivalic acid (t-BuC(O)OH) is PivOH.
The Rubottom oxidation is a useful, high-yielding chemical reaction between silyl enol ethers and peroxyacids to give the corresponding α-hydroxy carbonyl product. The mechanism of the reaction was proposed in its original disclosure by A.G. Brook with further evidence later supplied by George M. Rubottom. After a Prilezhaev-type oxidation of the silyl enol ether with the peroxyacid to form the siloxy oxirane intermediate, acid-catalyzed ring-opening yields an oxocarbenium ion. This intermediate then participates in a 1,4-silyl migration to give an α-siloxy carbonyl derivative that can be readily converted to the α-hydroxy carbonyl compound in the presence of acid, base, or a fluoride source.
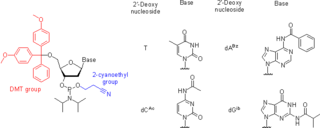
Nucleoside phosphoramidites are derivatives of natural or synthetic nucleosides. They are used to synthesize oligonucleotides, relatively short fragments of nucleic acid and their analogs. Nucleoside phosphoramidites were first introduced in 1981 by Beaucage and Caruthers. To avoid undesired side reactions, reactive hydroxy and exocyclic amino groups present in natural or synthetic nucleosides are appropriately protected. As long as a nucleoside analog contains at least one hydroxy group, the use of the appropriate protecting strategy allows one to convert that to the respective phosphoramidite and to incorporate the latter into synthetic nucleic acids. To be incorporated in the middle of an oligonucleotide chain using phosphoramidite strategy, the nucleoside analog must possess two hydroxy groups or, less often, a hydroxy group and another nucleophilic group (amino or mercapto). Examples include, but are not limited to, alternative nucleotides, LNA, morpholino, nucleosides modified at the 2'-position (OMe, protected NH2, F), nucleosides containing non-canonical bases (hypoxanthine and xanthine contained in natural nucleosides inosine and xanthosine, respectively, tricyclic bases such as G-clamp, etc.) or bases derivatized with a fluorescent group or a linker arm.
Oligonucleotide synthesis is the chemical synthesis of relatively short fragments of nucleic acids with defined chemical structure (sequence). The technique is extremely useful in current laboratory practice because it provides a rapid and inexpensive access to custom-made oligonucleotides of the desired sequence. Whereas enzymes synthesize DNA and RNA only in a 5' to 3' direction, chemical oligonucleotide synthesis does not have this limitation, although it is most often carried out in the opposite, 3' to 5' direction. Currently, the process is implemented as solid-phase synthesis using phosphoramidite method and phosphoramidite building blocks derived from protected 2'-deoxynucleosides, ribonucleosides, or chemically modified nucleosides, e.g. LNA or BNA.

Tripotassium phosphate, also called tribasic potassium phosphate is a water-soluble salt with the chemical formula K3PO4.(H2O)x (x = 0, 3, 7, 9). Tripotassium phosphate is basic.

Pancratistatin (PST) is a natural compound initially extracted from spider lily, a Hawaiian native plant of the family Amaryllidaceae (AMD).
Masad J. Damha is a Canadian academic and nucleic acid researcher. He is Distinguished James McGill Professor of Chemistry at McGill University in Montreal, Quebec, Canada.
A photolabile protecting group is a chemical modification to a molecule that can be removed with light. PPGs enable high degrees of chemoselectivity as they allow researchers to control spatial, temporal and concentration variables with light. Control of these variables is valuable as it enables multiple PPG applications, including orthogonality in systems with multiple protecting groups. As the removal of a PPG does not require chemical reagents, the photocleavage of a PPG is often referred to as "traceless reagent processes", and is often used in biological model systems and multistep organic syntheses. Since their introduction in 1962, numerous PPGs have been developed and utilized in a variety of wide-ranging applications from protein science to photoresists. Due to the large number of reported protecting groups, PPGs are often categorized by their major functional group(s); three of the most common classifications are detailed below.
Mixed anion compounds, heteroanionic materials or mixed anion materials are chemical compounds containing cations and more than one kind of anion. The compounds contain a single phase, rather than just a mixture.
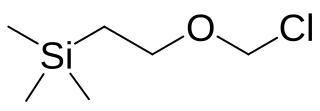
2-(Trimethylsilyl)ethoxymethyl chloride (SEM-Cl) is an organochlorine compound with the formula C6H15ClOSi, which was developed by Bruce H. Lipshutz during his work on the synthesis of N-methylmaysenine. It is used to protect hydroxyl group, which can be cleaved with fluoride in organic solvents selectively under mild conditions. Typically tetrabutylammonium fluoride and caesium fluoride can be used as deprotection reagents. Alternatives such as magnesium bromide, lithium tetrafluoroborate and boron trifluoride etherate were also developed to deprotect SEM group.
References
- ↑ Chmielewski, Marcin K. (2012). "Novel thermolabile protecting groups with higher stability at ambient temperature". Tetrahedron Letters. 53 (6): 666–669. doi:10.1016/j.tetlet.2011.11.122. ISSN 0040-4039.
- ↑ Chmielewski M.K., Marchán V., Cieślak J., Grajkowski A., Livengood V., Müchen U., Wilk A., Beaucage S.L., „Thermolytic carbonates for potential 5’-hydroxyl protection of deoxyribonucleosides” J. Org. Chem., 68, 10003-10012 (2003)
- ↑ Brzezinska, J.; Witkowska, A.; Bałabańska, S.; Chmielewski, M.K. „2-Pyridinyl-N-(2,4-difluorobenzyl)aminoethyl Group As Thermocontrolled Implement for Protection of Carboxylic Acids” Organic Letters 18, 3230–3233 (2016) DOI: 10.1021/acs.orglett.6b01475 (2016)
- ↑ Chmielewski M.K., “Protecting of a thermolabile protecting group: “Click-clack” approach” Organic Letters, 11 (16) 3742-3745 (2009) DOI: 10.1021/ol901358d
- ↑ Witkowska A., Krygier D., Brzezinska J., Chmielewski, M.K. „Modulating the Stability of 2-Pyridinyl Thermolabile Hydroxyl Protecting Groups via the “Chemical Switch” Approach” J. Org. Chem., 80, 12129−12136 (2015) DOI:10.1021/acs.joc.5b02033
- ↑ Grajkowski A.; Cieślak J., Chmielewski M.K., Marchán V., Phillips, L. R., and Beaucage, S. L., Wilk A.; „Conceptual “Heat-Driven” approach to the synthesis of DNA oligonucleotides on microarrays” Ann. N.Y. Acad. Sci., 1002, 1-11 (2003)