Antioxidants are compounds that inhibit oxidation. Oxidation is a chemical reaction that can produce free radicals, thereby leading to chain reactions that may damage the cells of organisms. Antioxidants such as thiols or ascorbic acid terminate these chain reactions. To balance the oxidative state, plants and animals maintain complex systems of overlapping antioxidants, such as glutathione and enzymes, produced internally, or the dietary antioxidants vitamin C, and vitamin E.
In chemistry, a disulfide refers to a functional group with the structure R−S−S−R′. The linkage is also called an SS-bond or sometimes a disulfide bridge and is usually derived by the coupling of two thiol groups. In biology, disulfide bridges formed between thiol groups in two cysteine residues are an important component of the secondary and tertiary structure of proteins. The connection is a persulfide, in analogy to its congener, peroxide (R−O−O−R′), but this terminology is rarely used, except in reference to hydrodisulfides.

Glutathione (GSH) is an antioxidant in plants, animals, fungi, and some bacteria and archaea. Glutathione is capable of preventing damage to important cellular components caused by reactive oxygen species such as free radicals, peroxides, lipid peroxides, and heavy metals. It is a tripeptide with a gamma peptide linkage between the carboxyl group of the glutamate side chain and the amine group of cysteine, and the carboxyl group of cysteine is attached by normal peptide linkage to a glycine.
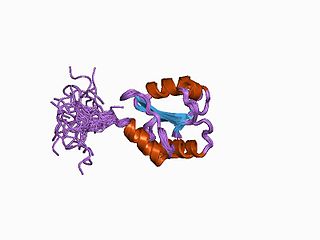
Protein disulfide isomerase, or PDI, is an enzyme in the endoplasmic reticulum (ER) in eukaryotes and the periplasm of bacteria that catalyzes the formation and breakage of disulfide bonds between cysteine residues within proteins as they fold. This allows proteins to quickly find the correct arrangement of disulfide bonds in their fully folded state, and therefore the enzyme acts to catalyze protein folding.

Glutathione disulfide (GSSG) is a disulfide derived from two glutathione molecules.

Glutathione reductase (GR) also known as glutathione-disulfide reductase (GSR) is an enzyme that in humans is encoded by the GSR gene. Glutathione reductase catalyzes the reduction of glutathione disulfide (GSSG) to the sulfhydryl form glutathione (GSH), which is a critical molecule in resisting oxidative stress and maintaining the reducing environment of the cell. Glutathione reductase functions as dimeric disulfide oxidoreductase and utilizes an FAD prosthetic group and NADPH to reduce one molar equivalent of GSSG to two molar equivalents of GSH:

Sulfur is an essential element for growth and physiological functioning of plants. However, its content strongly varies between plant species and it ranges from 0.1 to 6% of the plants' dry weight.

Glutaredoxins are small redox enzymes of approximately one hundred amino-acid residues that use glutathione as a cofactor. Glutaredoxins are oxidized by substrates, and reduced non-enzymatically by glutathione. In contrast to thioredoxins, which are reduced by thioredoxin reductase, no oxidoreductase exists that specifically reduces glutaredoxins. Instead, glutaredoxins are reduced by the oxidation of glutathione. Oxidized glutathione is then regenerated by glutathione reductase. Together these components compose the glutathione system.
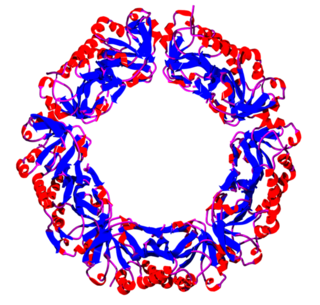
Peroxiredoxins are a ubiquitous family of antioxidant enzymes that also control cytokine-induced peroxide levels and thereby mediate signal transduction in mammalian cells. The family members in humans are PRDX1, PRDX2, PRDX3, PRDX4, PRDX5, and PRDX6. The physiological importance of peroxiredoxins is illustrated by their relative abundance.

Peroxiredoxin-2 is a protein that in humans is encoded by the PRDX2 gene.
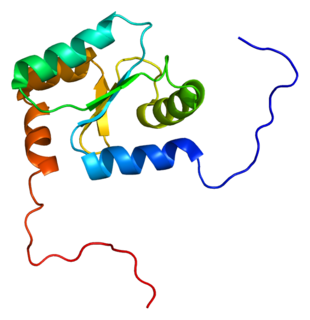
Glutaredoxin 2 (GLRX2) is an enzyme that in humans encoded by the GLRX2 gene. GLRX2, also known as GRX2, is a glutaredoxin family protein and a thiol-disulfide oxidoreductase that maintains cellular thiol homeostasis. This gene consists of four exons and three introns, spanned 10 kilobase pairs, and localized to chromosome 1q31.2–31.3.
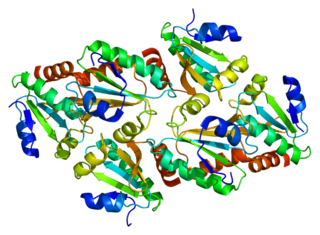
Thioredoxin, mitochondrial also known as thioredoxin-2 is a protein that in humans is encoded by the TXN2 gene on chromosome 22. This nuclear gene encodes a mitochondrial member of the thioredoxin family, a group of small multifunctional redox-active proteins. The encoded protein may play important roles in the regulation of the mitochondrial membrane potential and in protection against oxidant-induced apoptosis.
Thioredoxins are small disulfide-containing redox proteins that have been found in all the kingdoms of living organisms. Thioredoxin serves as a general protein disulfide oxidoreductase. It interacts with a broad range of proteins by a redox mechanism based on reversible oxidation of 2 cysteine thiol groups to a disulfide, accompanied by the transfer of 2 electrons and 2 protons. The net result is the covalent interconversion of a disulfide and a dithiol.

Ferredoxin-thioredoxin reductase EC 1.8.7.2, systematic name ferredoxin:thioredoxin disulfide oxidoreductase, is a [4Fe-4S] protein that plays an important role in the ferredoxin/thioredoxin regulatory chain. It catalyzes the following reaction:
Protein-methionine-S-oxide reductase is an enzyme with systematic name protein-L-methionine:thioredoxin-disulfide S-oxidoreductase. This enzyme catalyses the following chemical reaction
Glutathione amide-dependent peroxidase (EC 1.11.1.17) is an enzyme with systematic name glutathione amide:hydrogen-peroxide oxidoreductase. This enzyme catalyses the following chemical reaction
Oxidation response is stimulated by a disturbance in the balance between the production of reactive oxygen species and antioxidant responses, known as oxidative stress. Active species of oxygen naturally occur in aerobic cells and have both intracellular and extracellular sources. These species, if not controlled, damage all components of the cell, including proteins, lipids and DNA. Hence cells need to maintain a strong defense against the damage. The following table gives an idea of the antioxidant defense system in bacterial system.













