Related Research Articles

In organic chemistry, an alkane, or paraffin, is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in which all the carbon–carbon bonds are single. Alkanes have the general chemical formula CnH2n+2. The alkanes range in complexity from the simplest case of methane, where n = 1, to arbitrarily large and complex molecules, like pentacontane or 6-ethyl-2-methyl-5-(1-methylethyl) octane, an isomer of tetradecane.

In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or in the terminal position. Terminal alkenes are also known as α-olefins.

In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis.

Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms. Study of structure determines their structural formula. Study of properties includes physical and chemical properties, and evaluation of chemical reactivity to understand their behavior. The study of organic reactions includes the chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical study.

Naphthalene is an organic compound with formula C
10H
8. It is the simplest polycyclic aromatic hydrocarbon, and is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings. It is the main ingredient of traditional mothballs.

In organic chemistry, the cycloalkanes are the monocyclic saturated hydrocarbons. In other words, a cycloalkane consists only of hydrogen and carbon atoms arranged in a structure containing a single ring, and all of the carbon-carbon bonds are single. The larger cycloalkanes, with more than 20 carbon atoms are typically called cycloparaffins. All cycloalkanes are isomers of alkenes.
In organic chemistry, an alkyl group is an alkane missing one hydrogen. The term alkyl is intentionally unspecific to include many possible substitutions. An acyclic alkyl has the general formula of −CnH2n+1. A cycloalkyl group is derived from a cycloalkane by removal of a hydrogen atom from a ring and has the general formula −CnH2n−1. Typically an alkyl is a part of a larger molecule. In structural formulae, the symbol R is used to designate a generic (unspecified) alkyl group. The smallest alkyl group is methyl, with the formula −CH3.
In organic chemistry, a propyl group is a three-carbon alkyl substituent with chemical formula −CH2CH2CH3 for the linear form. This substituent form is obtained by removing one hydrogen atom attached to the terminal carbon of propane. A propyl substituent is often represented in organic chemistry with the symbol Pr.
In organic chemistry, a substituent is one or a group of atoms that replaces atoms, thereby becoming a moiety in the resultant (new) molecule.

In organic chemistry, spiro compounds are compounds that have at least two molecular rings with only one common atom. The simplest spiro compounds are bicyclic, or have a bicyclic portion as part of the larger ring system, in either case with the two rings connected through the defining single common atom. The one common atom connecting the participating rings distinguishes spiro compounds from other bicyclics: from isolated ring compounds like biphenyl that have no connecting atoms, from fused ring compounds like decalin having two rings linked by two adjacent atoms, and from bridged ring compounds like norbornane with two rings linked by two non-adjacent atoms.
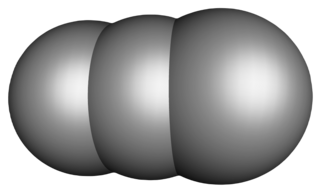
Tricarbon is an inorganic compound with the chemical formula C
2(μ-C). It is a colourless gas that only persists in dilution or solution as an adduct. It is one of the simplest unsaturated carbenes. Tricarbon can be found in interstellar space and can be produced in the laboratory by a process called laser ablation.

Fluoranthene is a polycyclic aromatic hydrocarbon (PAH). The molecule can be viewed as the fusion of naphthalene and benzene unit connected by a five-membered ring. Although samples are often pale yellow, the compound is colorless. It is soluble in nonpolar organic solvents. It is a member of the class of PAHs known as non-alternant PAHs because it has rings other than those with six carbon atoms. It is a structural isomer of the alternant PAH pyrene. It is not as thermodynamically stable as pyrene. Its name is derived from its fluorescence under UV light.

A cyclic compound is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where all the atoms are carbon, none of the atoms are carbon, or where both carbon and non-carbon atoms are present. Depending on the ring size, the bond order of the individual links between ring atoms, and their arrangements within the rings, carbocyclic and heterocyclic compounds may be aromatic or non-aromatic; in the latter case, they may vary from being fully saturated to having varying numbers of multiple bonds between the ring atoms. Because of the tremendous diversity allowed, in combination, by the valences of common atoms and their ability to form rings, the number of possible cyclic structures, even of small size numbers in the many billions.
In the field of organic chemistry, a polycyclic compound is an organic compound featuring several closed rings of atoms, primarily carbon. These ring substructures include cycloalkanes, aromatics, and other ring types. They come in sizes of three atoms and upward, and in combinations of linkages that include tethering, fusing, links via a single atom, bridged compounds, and longifolene. Though poly- literally means "many", there is some latitude in determining how many rings are required to be considered polycyclic; many smaller rings are described by specific prefixes, and so while it can refer to these, the title term is used with most specificity when these alternative names and prefixes are unavailable.

Cyclopropenylidene, or c-C3H2, is a partially aromatic molecule belonging to a highly reactive class of organic molecules known as carbenes. On Earth, cyclopropenylidene is only seen in the laboratory due to its reactivity. However, cyclopropenylidene is found in significant concentrations in the interstellar medium (ISM) and on Saturn's moon Titan. Its C2v symmetric isomer, propadienylidene (CCCH2) is also found in the ISM, but with abundances about an order of magnitude lower. A third C2 symmetric isomer, propargylene (HCCCH), has not yet been detected in the ISM, most likely due to its low dipole moment.
In organic chemistry, the carbon number of a compound is the number of carbon atoms in each molecule. The properties of hydrocarbons can be correlated with the carbon number, although the carbon number alone does not give an indication of the saturation of the organic compound. When describing a particular molecule, the "carbon number" is also the ordinal position of a particular carbon atom in a chain.

Four-carbon molecules are based on a skeleton made from four carbon atoms. They may be in a chain, branched chains, cycles or even bicyclic compounds
The C2benzenes are a class of organic aromatic compounds which contain a benzene ring and two other carbon atoms. For the hydrocarbons with no further unsaturation, there are four isomers. There are three xylenes and one ethylbenzene. The substances are:

The C3-benzenes are a class of organic aromatic compounds which contain a benzene ring and three other carbon atoms. For the hydrocarbons with no further unsaturation, there are four isomers. The chemical formula for all the saturated isomers is C9H12.

The C4-benzenes are a class of organic aromatic compounds which contain a benzene ring and four other carbon atoms. There are three tetramethylbenzenes, six dimethylethylbenzenes, three diethylbenzenes, three isopropylmethylbenzenes, three n-propylmethylbenzenes and four butylbenzenes. The saturated compounds have formula C10H14 and molecular weight 134.22 g/mol. C4-benzenes are found in petroleum. Petrol (gasoline) can contain 5-8% C4-benzenes.
References
- ↑ "Substance Information - ECHA Hydrocarbons, C3". echa.europa.eu.