Related Research Articles

A mitochondrion is a double membrane-bound organelle found in most eukaryotic organisms. Mitochondria generate most of the cell's supply of adenosine triphosphate (ATP), used as a source of chemical energy. Mitochondria were first discovered by Kolliker(1880 AD) in the voluntary muscles of insects. A mitochondrion is thus nicknamed the powerhouse of the cell, first coined by Philip Siekevitz in a 1957 article of the same name.
Protein targeting or protein sorting is the biological mechanism by which proteins are transported to their appropriate destinations within or outside the cell. Proteins can be targeted to the inner space of an organelle, different intracellular membranes, the plasma membrane, or to the exterior of the cell via secretion. Information contained in the protein itself directs this delivery process. Correct sorting is crucial for the cell; errors have been linked to multiple disease-states.
Protein disulfide isomerase, or PDI, is an enzyme in the endoplasmic reticulum (ER) in eukaryotes and the periplasm of bacteria that catalyzes the formation and breakage of disulfide bonds between cysteine residues within proteins as they fold. This allows proteins to quickly find the correct arrangement of disulfide bonds in their fully folded state, and therefore the enzyme acts to catalyze protein folding.

The intermembrane space (IMS) is the space occurring between or involving two or more membranes. In cell biology, it is most commonly described as the region between the inner membrane and the outer membrane of a mitochondrion or a chloroplast. It also refers to the space between the inner and outer nuclear membranes of the nuclear envelope, but is often called the perinuclear space. The IMS of mitochondria plays a crucial role in coordinating a variety of cellular activities, such as regulation of respiration and metabolic functions. Unlike the IMS of the mitochondria, the IMS of the chloroplast does not seem to have any obvious function.
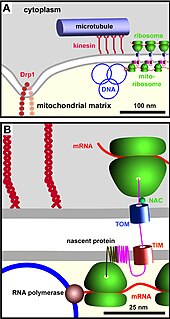
The TIM/TOM complex is a protein complex in cellular biochemistry which translocates proteins produced from nuclear DNA through the mitochondrial membrane for use in oxidative phosphorylation. In enzymology, the complex is described as an mitochondrial protein-transporting ATPase, or more systematically ATP phosphohydrolase , as the TIM part requires ATP hydrolysis to work.

Mitochondrial membrane transport proteins, also known as mitochondrial carrier proteins, are proteins which exist in the membranes of mitochondria. They serve to transport molecules and other factors, such as ions, into or out of the organelles. Mitochondria contain both an inner and outer membrane, separated by the inter-membrane space, or inner boundary membrane. The outer membrane is porous, whereas the inner membrane restricts the movement of all molecules. The two membranes also vary in membrane potential and pH. These factors play a role in the function of mitochondrial membrane transport proteins. There are 53 discovered human mitochondrial membrane transporters, with many others that are known to still need discovered.

Cytochromes c cytochromes, or heme-containing proteins, that have heme C covalently attached to the peptide backbone via one or two thioether bonds. These bonds are in most cases part of a specific Cys-X-X-Cys-His (CXXCH) binding motif, where X denotes a miscellaneous amino acid. Two thioether bonds of cysteine residues bind to the vinyl sidechains of heme, and the histidine residue coordinates one axial binding site of the heme iron. Less common binding motifs can include a single thioether linkage, a lysine or a methionine instead of the axial histidine or a CXnCH binding motif with n>2. The second axial site of the iron can be coordinated by amino acids of the protein, substrate molecules or water. Cytochromes c possess a wide range of properties and function as electron transfer proteins or catalyse chemical reactions involving redox processes. A prominent member of this family is mitochondrial cytochrome c.

Mitochondrial import inner membrane translocase subunit Tim8 A, also known as Deafness-dystonia peptide or protein is an enzyme that in humans is encoded by the TIMM8A gene. This translocase has similarity to yeast mitochondrial proteins that are involved in the import of metabolite transporters from the cytoplasm into the mitochondrial inner membrane. The gene is mutated in Deafness-dystonia syndrome and it is postulated that MTS/DFN-1 is a mitochondrial disease caused by a defective mitochondrial protein import system.

Mitochondrial import receptor subunit TOM20 homolog is a protein that in humans is encoded by the TOMM20 gene.

Mitochondrial import receptor subunit TOM22 homolog is a protein that in humans is encoded by the TOMM22 gene.

Mitochondrial import inner membrane translocase subunit Tim13 is an enzyme that in humans is encoded by the TIMM13 gene.

The translocase of the outer membrane (TOM) is a complex of proteins found in the outer mitochondrial membrane of the mitochondria. It allows movement of proteins through this barrier and into the intermembrane space of the mitochondrion. Most of the proteins needed for mitochondrial function are encoded by the nucleus of the cell. The outer membrane of the mitochondrion is impermeable to large molecules greater than 5000 Daltons. The TOM works in conjunction with the translocase of the inner membrane (TIM) to translocate proteins into the mitochondrion. Many of the proteins in the TOM complex, such as TOMM22, were first identified in Neurospora crassa and Saccharomyces cerevisiae.
Mitochondrial import inner membrane translocase subunit Tim10 is an enzyme that in humans is encoded by the TIMM10 gene.

Mitochondrial import inner membrane translocase subunit Tim9 B is an enzyme that in humans is encoded by the FXC1 gene.

Mitochondrial import receptor subunit TOM70 is a protein that in humans is encoded by the TOMM70A gene.

Mitochondrial import inner membrane translocase subunit Tim9 is an enzyme that in humans is encoded by the TIMM9 gene.
The translocase of the inner membrane (TIM) is a complex of proteins found in the inner mitochondrial membrane of the mitochondria. Components of the TIM complex facilitate the translocation of proteins across the inner membrane and into the mitochondrial matrix. They also facilitate the insertion of proteins into the inner mitochondrial membrane, where they must reside in order to function, these mainly include members of the mitochondrial carrier family of proteins.
The outer mitochondrial membrane is made up of two essential proteins, Tom40 and Sam50.
Copper chaperone for superoxide dismutase is a metalloprotein that is responsible for the delivery of Cu to superoxide dismutase (SOD1). CCS is a 54kDa protein that is present in mammals and most eukaryotes including yeast. The structure of CCS is composed of three distinct domains that are necessary for its function. Although CCS is important for many organisms, there are CCS independent pathways for SOD1, and many species lack CCS all together, such as C. elegans. In humans the protein is encoded by the CCS gene.

GFER Syndrome is a rare mitochondrial disease. GFER was first reported in 2009 and since Exome sequencing became more available, few more cases were discovered. In all known cases, the disease progresses with conditions that include: congenital cataracts, loss of motor abilities, development delay, degeneration of organs, sometimes hearing loss, etc.
References
- 1 2 Webb, CT; Gorman, MA; Lazarou, M; Ryan, MT; Gulbis, JM (6 January 2006). "Crystal structure of the mitochondrial chaperone TIM9.10 reveals a six-bladed alpha-propeller". Molecular Cell. 21 (1): 123–33. doi:10.1016/j.molcel.2005.11.010. PMID 16387659 . Retrieved 16 April 2021.
- 1 2 3 Mesecke, N; Terziyska, N; Kozany, C; Baumann, F; Neupert, W; Hell, K; Herrmann, JM (1 July 2005). "A disulfide relay system in the intermembrane space of mitochondria that mediates protein import". Cell. 121 (7): 1059–69. doi:10.1016/j.cell.2005.04.011. PMID 15989955. S2CID 11880968 . Retrieved 16 April 2021.
- ↑ Chacinska, A; Pfannschmidt, S; Wiedemann, N; Kozjak, V; Sanjuán Szklarz, LK; Schulze-Specking, A; Truscott, KN; Guiard, B; Meisinger, C; Pfanner, N (1 October 2004). "Essential role of Mia40 in import and assembly of mitochondrial intermembrane space proteins". The EMBO Journal. 23 (19): 3735–46. doi:10.1038/sj.emboj.7600389. PMC 522791 . PMID 15359280.
- ↑ Tokatlidis, K (1 July 2005). "A disulfide relay system in mitochondria". Cell. 121 (7): 965–7. doi:10.1016/j.cell.2005.06.019. PMID 15989945. S2CID 16851304 . Retrieved 16 April 2021.
- ↑ Curran, SP; Leuenberger, D; Leverich, EP; Hwang, DK; Beverly, KN; Koehler, CM (15 October 2004). "The role of Hot13p and redox chemistry in the mitochondrial TIM22 import pathway". The Journal of Biological Chemistry. 279 (42): 43744–51. doi: 10.1074/jbc.M404878200 . PMID 15294910.