Related Research Articles

An oxide is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. Metal oxides thus typically contain an anion of oxygen in the oxidation state of −2. Most of the Earth's crust consists of solid oxides, the result of elements being oxidized by the oxygen in air or in water. Even materials considered pure elements often develop an oxide coating. For example, aluminium foil develops a thin skin of Al2O3 (called a passivation layer) that protects the foil from further corrosion. Certain elements can form multiple oxides, differing in the amounts of the element combining with the oxygen. Examples are carbon, iron, nitrogen (see nitrogen oxide), silicon, titanium, lithium, and aluminium. In such cases the oxides are distinguished by specifying the numbers of atoms involved, as in carbon monoxide and carbon dioxide, or by specifying the element's oxidation number, as in iron(II) oxide and iron(III) oxide.

Rutile is an oxide mineral composed primarily of titanium dioxide (TiO2), the most common natural form of TiO2. Rarer polymorphs of TiO2 are known, including anatase, akaogiite, and brookite.

Titanium dioxide, also known as titanium(IV) oxide or titania, is the inorganic compound with the chemical formula TiO
2. When used as a pigment, it is called titanium white, Pigment White 6 (PW6), or CI 77891. It is a white, water-insoluble solid, although mineral forms can appear black. As a pigment, it has a wide range of applications, including paint, sunscreen, and food coloring. When used as a food coloring, it has E number E171. World production in 2014 exceeded 9 million tonnes. It has been estimated that titanium dioxide is used in two-thirds of all pigments, and pigments based on the oxide have been valued at $13.2 billion.
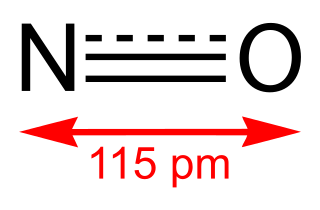
Nitric oxide is a colorless gas with the formula NO. It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its chemical formula. Nitric oxide is also a heteronuclear diatomic molecule, a class of molecules whose study spawned early modern theories of chemical bonding.

Nitrogen dioxide is a chemical compound with the formula NO
2. It is one of several nitrogen oxides. NO
2 is an intermediate in the industrial synthesis of nitric acid, millions of tons of which are produced each year for use primarily in the production of fertilizers. At higher temperatures it is a reddish-brown gas. It can be fatal if inhaled in large quantity. Nitrogen dioxide is a paramagnetic, bent molecule with C2v point group symmetry.
An acidic oxide is an oxide that either produces an acidic solution upon addition to water, or acts as an acceptor of hydroxide ions effectively functioning as a Lewis acid. Acidic oxides will typically have a low pKa and may be inorganic or organic. A commonly encountered acidic oxide, carbon dioxide produces an acidic solution when dissolved.
A "photoelectrochemical cell" is one of two distinct classes of device. The first produces electrical energy similarly to a dye-sensitized photovoltaic cell, which meets the standard definition of a photovoltaic cell. The second is a photoelectrolytic cell, that is, a device which uses light incident on a photosensitizer, semiconductor, or aqueous metal immersed in an electrolytic solution to directly cause a chemical reaction, for example to produce hydrogen via the electrolysis of water.

An air purifier or air cleaner is a device which removes contaminants from the air in a room to improve indoor air quality. These devices are commonly marketed as being beneficial to allergy sufferers and asthmatics, and at reducing or eliminating second-hand tobacco smoke.

Tungsten(VI) oxide, also known as tungsten trioxide or tungstic anhydride, WO3, is a chemical compound containing oxygen and the transition metal tungsten. It is obtained as an intermediate in the recovery of tungsten from its minerals. Tungsten ores are treated with alkalis to produce WO3. Further reaction with carbon or hydrogen gas reduces tungsten trioxide to the pure metal.

In chemistry, photocatalysis is the acceleration of a photoreaction in the presence of a catalyst. In catalysed photolysis, light is absorbed by an adsorbed substrate. In photogenerated catalysis, the photocatalytic activity (PCA) depends on the ability of the catalyst to create electron–hole pairs, which generate free radicals (e.g. hydroxyl radicals: •OH) able to undergo secondary reactions. Its practical application was made possible by the discovery of water electrolysis by means of titanium dioxide (TiO2).
Photo-catalytic concrete is a formulation of concrete used as pavers and other structural concrete that includes titanium dioxide (TiO2) as an admixture or superficial layer. Titanium dioxide is a heterogeneous photocatalyst that uses sunlight and moisture to absorb and render oxides of nitrogen (NO and NO2) into nitrate ions (NO3−), which are then either washed away by rain or soaked into the concrete to form stable compounds.
Self-cleaning glass is a specific type of glass with a surface that keeps itself free of dirt and grime.
Advanced oxidation processes (AOPs), in a broad sense, are a set of chemical treatment procedures designed to remove organic (and sometimes inorganic) materials in water and wastewater by oxidation through reactions with hydroxyl radicals (·OH). In real-world applications of wastewater treatment, however, this term usually refers more specifically to a subset of such chemical processes that employ ozone (O3), hydrogen peroxide (H2O2) and/or UV light. One such type of process is called in situ chemical oxidation.
The environmental impact of concrete, its manufacture and applications, are complex. Some effects are harmful; others welcome. Many depend on circumstances. A major component of concrete is cement, which has its own environmental and social impacts and contributes largely to those of concrete.
A photocatalyst activity indicator ink (Paii) is a substance used to identify the presence of an underlying heterogeneous photocatalyst and to measure its activity. Such inks render visible the activity of photocatalytic coatings applied to various "self-cleaning" products. The inks contain a dyestuff that reacts to ultraviolet radiation in the presence of the photocatalytic agent in the coating. They are applied to the coated product and show a color change or disappearance when exposed to ultraviolet radiation. The use of a Paii based on the dye resazurin forms the basis of an ISO standard test for photocatalytic activity.

Synthetic dyes are found in a wide range of products such as clothes, leather accessories, and furniture. These dyes are commonly used every day. However, a side effect of their widespread use is that up to 12% of these dyes are wasted during the dying process and about 20% of this wastage enters the environment.
Micromotors are very small particles that can move themselves. The term is often used interchangeably with "nanomotor," despite the implicit size difference. These micromotors actually propel themselves in a specific direction autonomously when placed in a chemical solution. There are many different micromotor types operating under a host of mechanisms. Easily the most important examples are biological motors such as bacteria and any other self-propelled cells. Synthetically, researchers have exploited oxidation-reduction reactions to produce chemical gradients, local fluid flows, or streams of bubbles that then propel these micromotors through chemical media.

Photogeochemistry merges photochemistry and geochemistry into the study of light-induced chemical reactions that occur or may occur among natural components of Earth's surface. The first comprehensive review on the subject was published in 2017 by the chemist and soil scientist Timothy A Doane, but the term photogeochemistry appeared a few years earlier as a keyword in studies that described the role of light-induced mineral transformations in shaping the biogeochemistry of Earth; this indeed describes the core of photogeochemical study, although other facets may be admitted into the definition.

Titanium dioxide nanoparticles, also called ultrafine titanium dioxide or nanocrystalline titanium dioxide or microcrystalline titanium dioxide, are particles of titanium dioxide with diameters less than 100 nm. Ultrafine TiO2 is used in sunscreens due to its ability to block ultraviolet radiation while remaining transparent on the skin. It is in rutile crystal structure and coated with silica or/and alumina to prevent photocatalytic phenomena. The health risks of ultrafine TiO2 from dermal exposure on intact skin are considered extremely low, and it is considered safer than other substances used for ultraviolet protection.

Kazuhito Hashimoto is a Japanese chemist. Between 2004 and 2007 he headed the Research Center for Advanced Science and Technology at the University of Tokyo, and since 2016 he serves as President of the National Institute for Materials Science (NIMS). He is the editor-in-chief of the journal Science and Technology of Advanced Materials.
References
- ↑ Bolte, G. (2005), "Photocatalysis in cement-bonded building materials". Cement International 03/2005 Vol. 3, pp. 92-97.
- ↑ Martin Hunger, Götz Hüsken, Jos Brouwers, "Photocatalysis applied to concrete products–part 1". ZKG international, No. 8-2008 (Volume 61), 77-85.
- ↑ UNI11247, Diterminazione dell’attività di degradazione di ossidi di azoto in aria de parte di materiali inirganic fotocatalytici (in Italian)
- 1 2 Bolte, G. (2009): Innovative building materials - reduction of pollutants with TioCem, ZKG international No. 1-2009, (Volume 62), 63-70
- ↑ PICADA Project Retrieved 2012-04-12.
- ↑ Guerrini, Gian Luca (2009), "Some observations regarding in-service performance". BFT 05/2009, 16-25.
- ↑ Ramondo, Valeriana (2007), "Galleria Umberto I a Roma". Progretto & Pubblico Dicembre 2007, 48-50 (in Italian).