
Hematite, also spelled as haematite, is a common iron oxide compound with the formula, Fe2O3 and is widely found in rocks and soils. Hematite crystals belong to the rhombohedral lattice system which is designated the alpha polymorph of Fe
2O
3. It has the same crystal structure as corundum (Al
2O
3) and ilmenite (FeTiO
3). With this it forms a complete solid solution at temperatures above 950 °C (1,740 °F).

Ore is natural rock or sediment that contains one or more valuable minerals, typically containing metals, that can be mined, treated and sold at a profit. Ore is extracted from the earth through mining and treated or refined, often via smelting, to extract the valuable metals or minerals. The grade of ore refers to the concentration of the desired material it contains. The value of the metals or minerals a rock contains must be weighed against the cost of extraction to determine whether it is of sufficiently high grade to be worth mining, and is therefore considered an ore.

Iron ores are rocks and minerals from which metallic iron can be economically extracted. The ores are usually rich in iron oxides and vary in color from dark grey, bright yellow, or deep purple to rusty red. The iron is usually found in the form of magnetite (Fe
3O
4, 72.4% Fe), hematite (Fe
2O
3, 69.9% Fe), goethite (FeO(OH), 62.9% Fe), limonite (FeO(OH)·n(H2O), 55% Fe) or siderite (FeCO3, 48.2% Fe).

Ilmenite is a titanium-iron oxide mineral with the idealized formula FeTiO
3. It is a weakly magnetic black or steel-gray solid. Ilmenite is the most important ore of titanium and the main source of titanium dioxide, which is used in paints, printing inks, fabrics, plastics, paper, sunscreen, food and cosmetics.

Magnetite is a mineral and one of the main iron ores, with the chemical formula Fe2+Fe3+2O4. It is one of the oxides of iron, and is ferrimagnetic; it is attracted to a magnet and can be magnetized to become a permanent magnet itself. With the exception of extremely rare native iron deposits, it is the most magnetic of all the naturally occurring minerals on Earth. Naturally magnetized pieces of magnetite, called lodestone, will attract small pieces of iron, which is how ancient peoples first discovered the property of magnetism.

Wüstite (FeO) is a mineral form of iron(II) oxide found with meteorites and native iron. It has a grey colour with a greenish tint in reflected light. Wüstite crystallizes in the isometric-hexoctahedral crystal system in opaque to translucent metallic grains. It has a Mohs hardness of 5 to 5.5 and a specific gravity of 5.88. Wüstite is a typical example of a non-stoichiometric compound.

Maghemite (Fe2O3, γ-Fe2O3) is a member of the family of iron oxides. It has the same spinel ferrite structure as magnetite and is also ferrimagnetic. It is sometimes spelled as "maghaemite".

Iron(II,III) oxide is the chemical compound with formula Fe3O4. It occurs in nature as the mineral magnetite. It is one of a number of iron oxides, the others being iron(II) oxide (FeO), which is rare, and iron(III) oxide (Fe2O3) which also occurs naturally as the mineral hematite. It contains both Fe2+ and Fe3+ ions and is sometimes formulated as FeO ∙ Fe2O3. This iron oxide is encountered in the laboratory as a black powder. It exhibits permanent magnetism and is ferrimagnetic, but is sometimes incorrectly described as ferromagnetic. Its most extensive use is as a black pigment. For this purpose, it is synthesized rather than being extracted from the naturally occurring mineral as the particle size and shape can be varied by the method of production.
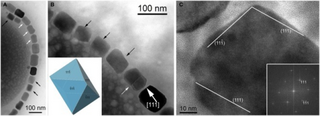
Magnetosomes are membranous structures present in magnetotactic bacteria (MTB). They contain iron-rich magnetic particles that are enclosed within a lipid bilayer membrane. Each magnetosome can often contain 15 to 20 magnetite crystals that form a chain which acts like a compass needle to orient magnetotactic bacteria in geomagnetic fields, thereby simplifying their search for their preferred microaerophilic environments. Recent research has shown that magnetosomes are invaginations of the inner membrane and not freestanding vesicles. Magnetite-bearing magnetosomes have also been found in eukaryotic magnetotactic algae, with each cell containing several thousand crystals.
In chemistry, a mixed oxide is a somewhat informal name for an oxide that contains cations of more than one chemical element or cations of a single element in several states of oxidation.

Chondrodite is a nesosilicate mineral with formula (Mg,Fe)
5(SiO
4)
2(F,OH,O)
2. Although it is a fairly rare mineral, it is the most frequently encountered member of the humite group of minerals. It is formed in hydrothermal deposits from locally metamorphosed dolomite. It is also found associated with skarn and serpentinite. It was discovered in 1817 at Pargas in Finland, and named from the Greek for "granule", which is a common habit for this mineral.

Ulvöspinel or ulvite is an iron titanium oxide mineral with formula: Fe2TiO4 or TiFe2+2O4. It forms brown to black metallic isometric crystals with a Mohs hardness of 5.5 to 6. It belongs to the spinel group of minerals, as does magnetite, Fe3O4.
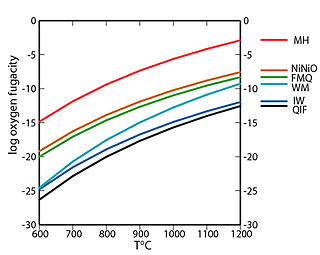
In geology, a redox buffer is an assemblage of minerals or compounds that constrains oxygen fugacity as a function of temperature. Knowledge of the redox conditions (or equivalently, oxygen fugacities) at which a rock forms and evolves can be important for interpreting the rock history. Iron, sulfur, and manganese are three of the relatively abundant elements in the Earth's crust that occur in more than one oxidation state. For instance, iron, the fourth most abundant element in the crust, exists as native iron, ferrous iron (Fe2+), and ferric iron (Fe3+). The redox state of a rock affects the relative proportions of the oxidation states of these elements and hence may determine both the minerals present and their compositions. If a rock contains pure minerals that constitute a redox buffer, then the oxygen fugacity of equilibration is defined by one of the curves in the accompanying fugacity-temperature diagram.

Cafetite is a rare titanium oxide mineral with formula (Ca,Mg)(Fe,Al)
2Ti
4O
12·4(H
2O). It is named for its composition, Ca-Fe-Ti.
Cleusonite is a member of the crichtonite group of minerals with the chemical formula (Pb,Sr)(U4+
,U6+
)(Fe2+
,Zn)
2(Ti,Fe2+
,Fe3+
)
18(O,OH)
38. This group of minerals contains approximately thirteen complex metal titanates. The structures of minerals of this group is complicated by frequent fine-scale twinning and metamictization due to radioactive elements. The crichtonite group consists of members of related mineral species of the type A{BC2D6E12}O38 which are characterized by their predominant cations (as seen in crichtonite (Sr), senaite (Pb), davidite (REE + U), landauite (Na), loveringite (Ca), lindsleyite (Ba), and mathiasite (K).
Magnetic mineralogy is the study of the magnetic properties of minerals. The contribution of a mineral to the total magnetism of a rock depends strongly on the type of magnetic order or disorder. Magnetically disordered minerals contribute a weak magnetism and have no remanence. The more important minerals for rock magnetism are the minerals that can be magnetically ordered, at least at some temperatures. These are the ferromagnets, ferrimagnets and certain kinds of antiferromagnets. These minerals have a much stronger response to the field and can have a remanence.

Pseudobrookite is an iron titanium oxide mineral with formula: Fe2TiO5 or (Fe3+,Fe2+)2(Ti,Fe2+)O5.

Dorrite is a silicate mineral that is isostructural to the aenigmatite group. Although it is most chemically similar to the mineral rhönite [Ca2Mg5Ti(Al2Si4)O20], the lack of titanium (Ti) and presence of Fe3+ influenced dorrite's independence. Dorrite is named for Dr. John (Jack) A. Dorr, a late professor at the University of Michigan that researched in outcrops where dorrite was found in 1982. This mineral is sub-metallic resembling colors of brownish-black, dark brown, to reddish brown.

A field is a mineral deposit containing a metal or other valuable resources in a cost-competitive concentration. It is usually used in the context of a mineral deposit from which it is convenient to extract its metallic component. The deposits are exploited by mining in the case of solid mineral deposits and extraction wells in case of fluids.

Nelsonite is an igneous rock primarily constituted of ilmenite and apatite, with anatase, chlorite, phosphosiderite, talc and/or wavellite appearing as minor components. Rocks are equigranular with a grain size around 2 - 3 mm. The black ilmenite is slightly magnetic while the whitish apatite is not.

















