In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs, often through Lewis bases. The nature of metal–ligand bonding can range from covalent to ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acidic "ligands".
Polysulfides are a class of chemical compounds containing chains of sulfur atoms. There are two main classes of polysulfides: inorganic and organic. Among the inorganic polysulfides, there are ones which contain anions, which have the general formula S2−
n. These anions are the conjugate bases of the hydrogen polysulfides H2Sn. Organic polysulfides generally have the formulae R1SnR2, where R = alkyl or aryl.

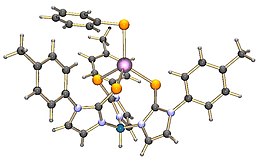
The term scorpionate ligand refers to a tridentate (three-donor-site) ligand which would bind to a metal in a fac manner. The most popular class of scorpionates are the hydrotris(pyrazolyl)borates or Tp ligands. These were also the first to become popular. These ligands first appeared in journals in 1966 from the then little-known DuPont chemist of Ukrainian descent, Swiatoslaw Trofimenko. Trofimenko called this discovery "a new and fertile field of remarkable scope".

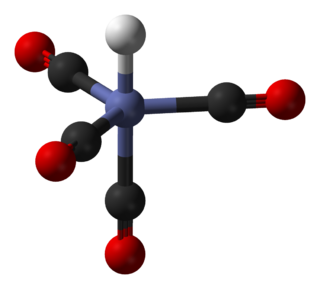
Cyanate is an anion with the structural formula [O=C=N]−, usually written OCN−. It also refers to any salt containing it, such as ammonium cyanate.

Sodium dithionite is a white crystalline powder with a sulfurous odor. Although it is stable in dry air, it decomposes in hot water and in acid solutions.
Pyrazole is an organic compound with the formula C3H3N2H. It is a heterocycle characterized by a 5-membered ring of three carbon atoms and two adjacent nitrogen atoms, which are in ortho-substitution. Pyrazole is a weak base, with pKb 11.5 (pKa of the conjugate acid 2.49 at 25 °C). Pyrazoles are also a class of compounds that have the ring C3N2 with adjacent nitrogen atoms. Notable drugs containing a pyrazole ring are celecoxib (celebrex) and the anabolic steroid stanozolol.

A persistent carbene (also known as stable carbene) is a type of carbene demonstrating particular stability. The best-known examples and by far largest subgroup are the N-heterocyclic carbenes (NHC) (sometimes called Arduengo carbenes), for example diaminocarbenes with the general formula (R2N)2C:, where the four R moieties are typically alkyl and aryl groups. The groups can be linked to give heterocyclic carbenes, such as those derived from imidazole, imidazoline, thiazole or triazole.

The trispyrazolylborate ligand, abbreviated Tp−, is an anionic tridentate and tripodal ligand. Trispyrazolylborate refers specifically to the anion [HB(C3N2H3)3]−, but the term trispyrazolylborate refers to derivatives substituted at on the pyrazolyl rings. This family of compounds are sometimes called scorpionate ligands.
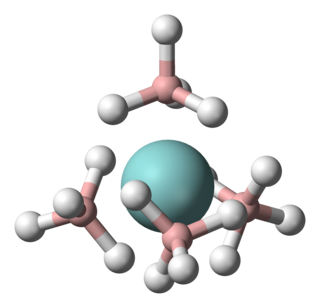
Uranium borohydride is the inorganic compound with the empirical formula U(BH4)4. Two polymeric forms are known, as well as a monomeric derivative that exists in the gas phase. Because the polymers convert to the gaseous form at mild temperatures, uranium borohydride once attracted much attention. It is solid green.

Borohydride refers to the anion [BH4]−, which is also called tetrahydroborate, and its salts. Borohydride or hydroborate is also the term used for compounds containing [BH4−nXn]−, where n is an integer from 0 to 3, for example cyanoborohydride or cyanotrihydroborate [BH3(CN)]− and triethylborohydride or triethylhydroborate [BH(CH2CH3)3]−. Borohydrides find wide use as reducing agents in organic synthesis. The most important borohydrides are lithium borohydride and sodium borohydride, but other salts are well known. Tetrahydroborates are also of academic and industrial interest in inorganic chemistry.

Metal nitrosyl complexes are complexes that contain nitric oxide, NO, bonded to a transition metal. Many kinds of nitrosyl complexes are known, which vary both in structure and coligand.

In organic chemistry, a dithiocarbamate is a functional group with the general formula R2NC(S)SR and structure >N−C(=S)−S−. It is the analog of a carbamate in which both oxygen atoms are replaced by sulfur atoms.

8-Hydroxyquinoline is an organic compound derived from the heterocycle quinoline. A colorless solid, its conjugate base is a chelating agent, which one was used for the quantitative determination of metal ions.
In coordination chemistry, a stability constant is an equilibrium constant for the formation of a complex in solution. It is a measure of the strength of the interaction between the reagents that come together to form the complex. There are two main kinds of complex: compounds formed by the interaction of a metal ion with a ligand and supramolecular complexes, such as host–guest complexes and complexes of anions. The stability constant(s) provide(s) the information required to calculate the concentration(s) of the complex(es) in solution. There are many areas of application in chemistry, biology and medicine.
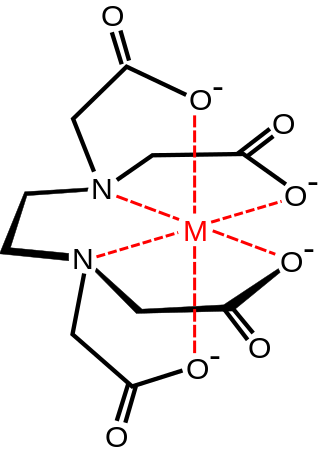
An aminopolycarboxylic acid is a chemical compound containing one or more nitrogen atoms connected through carbon atoms to two or more carboxyl groups. Aminopolycarboxylates that have lost acidic protons form strong complexes with metal ions. This property makes aminopolycarboxylic acids useful complexone in a wide variety of chemical, medical, and environmental applications.

The Kläui ligand is the anion {(C5H5)Co[(CH3O)2PO]3}−. The ligand, popularized by Wolfgang Kläui, binds metals and metalloids via a facial O3 donor set. Related tridentate and tripodal anionic ligands include trispyrazolylborates.

Potassium tris(3,5-dimethyl-1-pyrazolyl)borate, abbreviated KTp*, is the potassium salt of the anion HB((CH3)2C3N2H)3. Tp*− is a tripodal ligand that binds to a metal in a facial manner, more specifically a Scorpionate ligand. KTp* is a white crystalline solid that is soluble in polar solvents, including water and several alcohols.

Pyrithione is the common name of an organosulfur compound with molecular formula C
5H
5NOS, chosen as an abbreviation of pyridinethione, and found in the Persian shallot. It exists as a pair of tautomers, the major form being the thione 1-hydroxy-2(1H)-pyridinethione and the minor form being the thiol 2-mercaptopyridine N-oxide; it crystallises in the thione form. It is usually prepared from either 2-bromopyridine, 2-chloropyridine, or 2-chloropyridine N-oxide, and is commercially available as both the neutral compound and its sodium salt. It is used to prepare zinc pyrithione, which is used primarily to treat dandruff and seborrhoeic dermatitis in medicated shampoos, though is also an anti-fouling agent in paints.

A tridentate ligand is a ligand that has three atoms that can function as acceptor atoms in a coordination complex.
Transition metal amino acid complexes are a large family of coordination complexes containing the conjugate bases of the amino acids, the 2-aminocarboxylates. Amino acids are prevalent in nature, and all of them function as ligands toward the transition metals. Not included in this article are complexes of the amides and ester derivatives of amino acids. Also excluded are the polyamino acids including the chelating agents EDTA and NTA.