In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs, often through Lewis bases. The nature of metal–ligand bonding can range from covalent to ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acidic "ligands".

A metallocene is a compound typically consisting of two cyclopentadienyl anions (C
5H−
5, abbreviated Cp) bound to a metal center (M) in the oxidation state II, with the resulting general formula (C5H5)2M. Closely related to the metallocenes are the metallocene derivatives, e.g. titanocene dichloride or vanadocene dichloride. Certain metallocenes and their derivatives exhibit catalytic properties, although metallocenes are rarely used industrially. Cationic group 4 metallocene derivatives related to [Cp2ZrCH3]+ catalyze olefin polymerization.

A cyclopentadienyl complex is a coordination complex of a metal and cyclopentadienyl groups. Cyclopentadienyl ligands almost invariably bind to metals as a pentahapto (η5-) bonding mode. The metal–cyclopentadienyl interaction is typically drawn as a single line from the metal center to the center of the Cp ring.
Pyrazole is an organic compound of azole group with the formula C3H3N2H. It is a heterocycle characterized by a 5-membered ring of three carbon atoms and two adjacent nitrogen atoms, which are in ortho-substitution. Pyrazole is a weak base, with pKb 11.5 (pKa of the conjugate acid 2.49 at 25 °C). Pyrazoles are also a class of compounds that have the ring C3N2 with adjacent nitrogen atoms. Notable drugs containing a pyrazole ring are celecoxib (celebrex) and the anabolic steroid stanozolol.

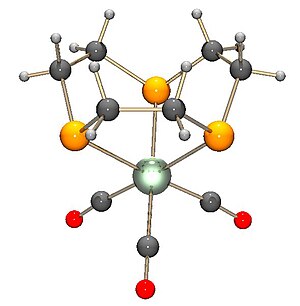
The trispyrazolylborate ligand, abbreviated Tp−, is an anionic tridentate and tripodal ligand. Trispyrazolylborate refers specifically to the anion [HB(C3N2H3)3]−. However, the term can also be used to refer to derivatives having substituents on the pyrazolyl rings. This class of compounds belongs to the family of ligands called scorpionate ligands.
Tm is an abbreviation for anionic tridentate ligand based on three imidazole-2-thioketone groups bonded to a borohydride center. They are examples of scorpionate ligands. Various ligands in this family are known, differing in what substituents are on the imidazoles. The most common is TmMe, which has a methyl group on the nitrogen. It is easily prepared by the reaction of molten methimazole (1-methylimidazole-2-thione) with sodium borohydride, giving the sodium salt of the ligand. Salts of the TmMe anion are known also for lithium and potassium. Other alkyl- and aryl-group variations are likewise named TmR according to those groups.

In coordination chemistry, hapticity is the coordination of a ligand to a metal center via an uninterrupted and contiguous series of atoms. The hapticity of a ligand is described with the Greek letter η ('eta'). For example, η2 describes a ligand that coordinates through 2 contiguous atoms. In general the η-notation only applies when multiple atoms are coordinated. In addition, if the ligand coordinates through multiple atoms that are not contiguous then this is considered denticity, and the κ-notation is used once again. When naming complexes care should be taken not to confuse η with μ ('mu'), which relates to bridging ligands.

1,1-Bis(diphenylphosphino)methane (dppm), is an organophosphorus compound with the formula CH2(PPh2)2. Dppm, a white, crystalline powder, is used in inorganic and organometallic chemistry as a ligand. It is more specifically a chelating ligand because it is a ligand that can bond to metals with two phosphorus donor atoms. The natural bite angle is 73°.
Nomenclature of Inorganic Chemistry, IUPAC Recommendations 2005 is the 2005 version of Nomenclature of Inorganic Chemistry. It is a collection of rules for naming inorganic compounds, as recommended by the International Union of Pure and Applied Chemistry (IUPAC).
Organovanadium chemistry is the chemistry of organometallic compounds containing a carbon (C) to vanadium (V) chemical bond. Organovanadium compounds find only minor use as reagents in organic synthesis but are significant for polymer chemistry as catalysts.

Rhodocene is a chemical compound with the formula [Rh(C5H5)2]. Each molecule contains an atom of rhodium bound between two planar aromatic systems of five carbon atoms known as cyclopentadienyl rings in a sandwich arrangement. It is an organometallic compound as it has (haptic) covalent rhodium–carbon bonds. The [Rh(C5H5)2] radical is found above 150 °C (302 °F) or when trapped by cooling to liquid nitrogen temperatures (−196 °C [−321 °F]). At room temperature, pairs of these radicals join via their cyclopentadienyl rings to form a dimer, a yellow solid.

Potassium trispyrazolylborate, commonly abbreviated KTp, is the potassium salt with the formula :KHB(C3N2H3)3. This salt is the source of the trispyrazolylborate ligand.
Tris(tert-butoxy)silanethiol is a silicon compound containing three tert-butoxy groups and a rare Si–S–H functional group. This colourless compound serves as an hydrogen donor in radical chain reactions. It was first prepared by alcoholysis of silicon disulfide and purified by distillation:

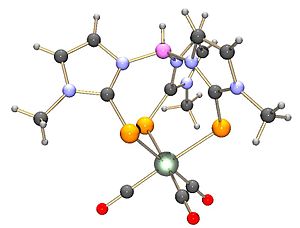
Potassium tris(3,5-dimethyl-1-pyrazolyl)borate, abbreviated KTp*, is the potassium salt of the anion HB((CH3)2C3N2H)3. Tp*− is a tripodal ligand that binds to a metal in a facial manner, more specifically a Scorpionate ligand. KTp* is a white crystalline solid that is soluble in polar solvents, including water and several alcohols.

Tris(oxazolinyl)borate compounds are a class of tridentate ligands; often abbreviated ToR, where R is the substituent on the oxazoline ring. Most commonly the substituent is either a methyl, propyl, tert-butyl or hydrogen. The formation of anionic boron backbone with addition of a phenyl group on boron allows the ligand to strongly bind to the metal center. It results in a more robust complex.

Transition-metal allyl complexes are coordination complexes with allyl and its derivatives as ligands. Allyl is the radical with the connectivity CH2CHCH2, although as a ligand it is usually viewed as an allyl anion CH2=CH−CH2−, which is usually described as two equivalent resonance structures.
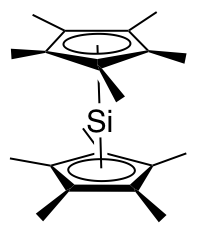
Decamethylsilicocene, (C5Me5)2Si, is a group 14 sandwich compound. It is an example of a main-group cyclopentadienyl complex; these molecules are related to metallocenes but contain p-block elements as the central atom. It is a colorless, air sensitive solid that sublimes under vacuum.

A lanthanocene is a type of metallocene compound that contains an element from the lanthanide series. The most common lanthanocene complexes contain two cyclopentadienyl anions and an X type ligand, usually hydride or alkyl ligand.

3,5-Dimethylpyrazole is an organic compound with the formula (CH3C)2CHN2H. It is one of several isomeric derivatives of pyrazole that contain two methyl substituents. The compound is unsymmetrical but the corresponding conjugate acid (pyrazolium) and conjugate base (pyrazolide) have C2v symmetry. It is a white solid that dissolves well in polar organic solvents.

Organoberyllium chemistry involves the synthesis and properties of organometallic compounds featuring the group 2 alkaline earth metal beryllium (Be). The area remains understudied, relative to the chemistry of other main-group elements, because although metallic beryllium is relatively unreactive, its dust causes berylliosis and compounds are toxic. Organoberyllium compounds are typically prepared by transmetallation or alkylation of beryllium chloride.