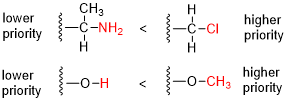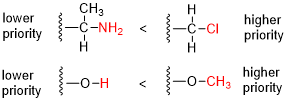
The Cahn–Ingold–Prelog (CIP) sequence rules, named for organic chemists Robert Sidney Cahn, Christopher Kelk Ingold, and Vladimir Prelog — alternatively termed the CIP priority rules, system, or conventions — are a standard process used in organic chemistry to completely and unequivocally name a stereoisomer of a molecule. The purpose of the CIP system is to assign an R or S descriptor to each stereocenter and an E or Z descriptor to each double bond so that the configuration of the entire molecule can be specified uniquely by including the descriptors in its systematic name. A molecule may contain any number of stereocenters and any number of double bonds, and each usually gives rise to two possible isomers. A molecule with an integer n describing the number of its stereogenic centers will usually have 2n stereoisomers, and 2n−1 diastereomers each having an associated pair of enantiomers. The CIP sequence rules contribute to the precise naming of every stereoisomer of every organic and organometallic molecule with all atoms of ligancy of fewer than 4.
Monosaccharides, also called simple sugars, are the simplest forms of sugar and the most basic units (monomers) from which all carbohydrates are built.
In chemistry, a structural isomer of a compound is another compound whose molecule has the same number of atoms of each element, but with logically distinct bonds between them. The term metamer was formerly used for the same concept.

In stereochemistry, stereoisomerism, or spatial isomerism, is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms in space. This contrasts with structural isomers, which share the same molecular formula, but the bond connections or their order differs. By definition, molecules that are stereoisomers of each other represent the same structural isomer.

In chemistry, a hexose is a monosaccharide (simple sugar) with six carbon atoms. The chemical formula for all hexoses is C6H12O6, and their molecular weight is 180.156 g/mol.
A carbanion is an anion in which carbon is trivalent and bears a formal negative charge.
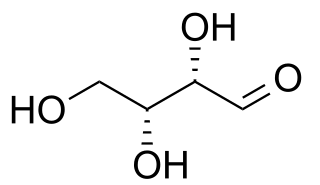
In stereochemistry, diastereomers are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have different configurations at one or more of the equivalent (related) stereocenters and are not mirror images of each other. When two diastereoisomers differ from each other at only one stereocenter they are epimers. Each stereocenter gives rise to two different configurations and thus typically increases the number of stereoisomers by a factor of two.

Diethyl malonate, also known as DEM, is the diethyl ester of malonic acid. It occurs naturally in grapes and strawberries as a colourless liquid with an apple-like odour, and is used in perfumes. It is also used to synthesize other compounds such as barbiturates, artificial flavourings, vitamin B1, and vitamin B6.

Unsaturated hydrocarbons are hydrocarbons that have double or triple covalent bonds between adjacent carbon atoms. The term "unsaturated" means more hydrogen atoms may be added to the hydrocarbon to make it saturated. The configuration of an unsaturated carbons include straight chain, such as alkenes and alkynes, as well as branched chains and aromatic compounds.

In chemistry, a molecule or ion is called chiral if it cannot be superposed on its mirror image by any combination of rotations, translations, and some conformational changes. This geometric property is called chirality. The terms are derived from Ancient Greek χείρ (cheir) 'hand'; which is the canonical example of an object with this property.

Enantioselective synthesis, also called asymmetric synthesis, is a form of chemical synthesis. It is defined by IUPAC as "a chemical reaction in which one or more new elements of chirality are formed in a substrate molecule and which produces the stereoisomeric products in unequal amounts."

In chemistry, octahedral molecular geometry describes the shape of compounds with six atoms or groups of atoms or ligands symmetrically arranged around a central atom, defining the vertices of an octahedron. The octahedron has eight faces, hence the prefix octa. The octahedron is one of the Platonic solids, although octahedral molecules typically have an atom in their centre and no bonds between the ligand atoms. A perfect octahedron belongs to the point group Oh. Examples of octahedral compounds are sulfur hexafluoride SF6 and molybdenum hexacarbonyl Mo(CO)6. The term "octahedral" is used somewhat loosely by chemists, focusing on the geometry of the bonds to the central atom and not considering differences among the ligands themselves. For example, [Co(NH3)6]3+, which is not octahedral in the mathematical sense due to the orientation of the N−H bonds, is referred to as octahedral.

In stereochemistry, asymmetric induction describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment. Asymmetric induction is a key element in asymmetric synthesis.

A chiral derivatizing agent (CDA) also known as a chiral resolving reagent, is a chiral auxiliary used to convert a mixture of enantiomers into diastereomers in order to analyze the quantities of each enantiomer present within the mix. Analysis can be conducted by spectroscopy or by chromatography. The use of chiral derivatizing agents has declined with the popularization of chiral HPLC. Besides analysis, chiral derivatization is also used for chiral resolution, the actual physical separation of the enantiomers.

Absolute configuration refers to the spatial arrangement of atoms within a chiral molecular entity and its resultant stereochemical description. Absolute configuration is typically relevant in organic molecules, where carbon is bonded to four different substituents. This type of construction creates two possible enantiomers. Absolute configuration uses a set of rules to describe the relative positions of each bond around the chiral center atom. The most common labeling method uses the descriptors R or S is based on the Cahn–Ingold–Prelog priority rules. R and S refer to Rectus and Sinister, which are Latin for right and left, respectively.

Pirkle's alcohol is an off-white, crystalline solid that is stable at room temperature when protected from light and oxygen. This chiral molecule is typically used, in nonracemic form, as a chiral shift reagent in nuclear magnetic resonance spectroscopy, in order to simultaneously determine absolute configuration and enantiomeric purity of other chiral molecules. The molecule is named after William H. Pirkle, Professor of Chemistry at the University of Illinois whose group reported its synthesis and its application as a chiral shift reagent.

In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers.
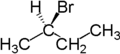
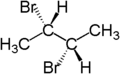
2-Bromobutane is an isomer of 1-bromobutane. Both compounds share the molecular formula C4H9Br. 2-Bromobutane is also known as sec-butyl bromide or methylethylbromomethane. Because it contains bromine, a halogen, it is part of a larger class of compounds known as alkyl halides. It is a colorless liquid with a pleasant odor. Because the carbon atom connected to the bromine is connected to two other carbons the molecule is referred to as a secondary alkyl halide. 2-Bromobutane is chiral and thus can be obtained as either of two enantiomers designated as (R)-(−)-2-bromobutane and (S)-(+)-2-bromobutane.

Dimethylphenylphosphine is an organophosphorus compound with a formula P(C6H5)(CH3)2. The phosphorus is connected to a phenyl group and two methyl groups, making it the simplest aromatic alkylphosphine. It is colorless air sensitive liquid. It is a member of series (CH3)3-n(C6H5)2P that also includes n = 0, n = 2, and n = 3 that are often employed as ligands in metal phosphine complexes.
Enantioselective ketone reductions convert prochiral ketones into chiral, non-racemic alcohols and are used heavily for the synthesis of stereodefined alcohols.