
The bag-1 internal ribosome entry site (IRES) is a cis-acting element located in the 5 ' untranslated region of the BAG-1 protein mRNA. Its effects apoptosis through IRES mediated translation of the BAG-1 protein.
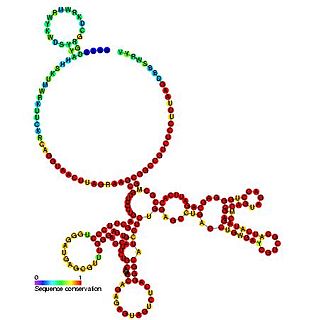
The BiP internal ribosome entry site (IRES) is an RNA element present in the 5' UTR of the mRNA of BiP protein and allows cap-independent translation. BiP protein expression has been found to be significantly enhanced by the heat shock response due to internal ribosome entry site (IRES)-dependent translation. It is thought that this translational mechanism is essential for the survival of cells under stress.
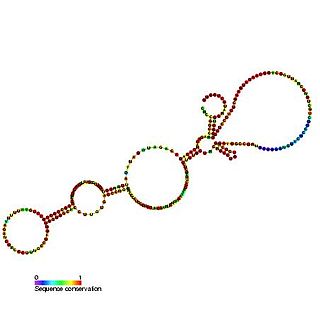
The c-myc internal ribosome entry site (IRES) is an RNA element present in the 5' UTR of the mRNA of C-myc and allows cap-independent translation. The mammalian c-myc gene is a proto-oncogene which is required for cell proliferation, transformation and death. c-myc mRNA has an alternative method of translation via internal ribosome entry where ribosomes are recruited to the IRES located in the 5' UTR thus bypassing the typical eukaryotic cap-dependent translation pathway.

The Cripavirus internal ribosome entry site is an RNA element required for the production of capsid proteins through ribosome recruitment to an intergenic region IRES.
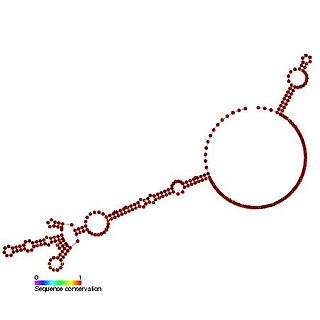
The c-sis internal ribosome entry site (IRES) is a RNA element found in the 5' UTR of the PDGF beta chain gene. The internal ribosome entry site contains three modules that can individually mediate internal ribosome entry. However, the full length sequence is required for maximal IRES activity. It is thought that the three IRES elements are somehow responsive to cellular changes and act to regulate the level of translation.
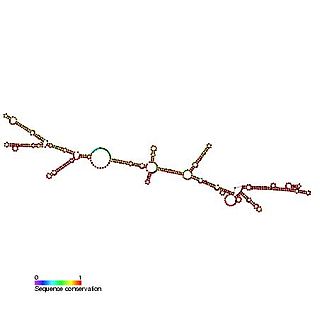
The Epstein–Barr virus nuclear-antigen internal ribosome entry site is an internal ribosome entry site (IRES) that is found in an exon in the 5' untranslated region of the Epstein–Barr virus nuclear antigen 1 (EBNA1) gene. The EBNA IRES allows EBNA1 translation to occur under situations where initiation from the 5' cap structure and ribosome scanning is reduced. It is thought that the EBNA IRES is necessary for the regulation of latent-gene expression.

The FGF-1 internal ribosome entry site (IRES) is an RNA element present in the 5' UTR of the mRNA of fibroblast growth factor-1 and allows cap-independent translation. It is thought that FGF-1 internal ribosome entry site (IRES) activity is strictly controlled and highly tissue specific.
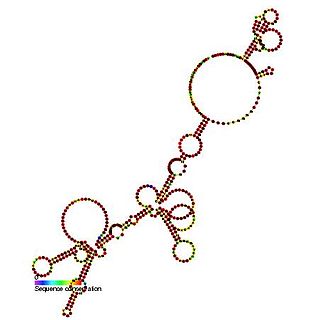
The FGF-2 internal ribosome entry site is an RNA element present in the 5' UTR of the mRNA of fibroblast growth factor-2. It has been found that the FGF-2 internal ribosome entry site (IRES) activity is strictly controlled and highly tissue specific. It is thought that translational IRES dependent activation of FGF-2 plays a vital role in embryogenesis and in the adult brain [1]. When expressed the fibroblast growth factor 2 FGF-2 protein plays a pivotal role in cell proliferation, differentiation and survival as well as being involved in wound-healing [1,2].
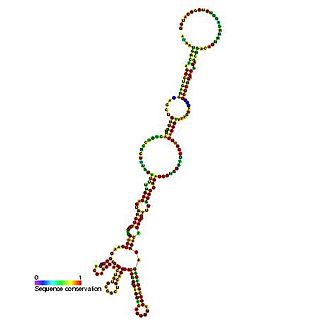
The heat shock protein 70 (Hsp70) internal ribosome entry site (IRES) is an RNA element that allows cap independent translation during conditions such as heat shock and stress. It has been shown that the 216 nucleotide long 5' UTR contains internal ribosome entry site activity.

The Hepatitis C virus internal ribosome entry site, or HCV IRES, is an RNA structure within the 5'UTR of the HCV genome that mediates cap-independent translation initiation.

The L-myc internal ribosome entry site (IRES) is an RNA element present in the 5' UTR of the mRNA of L-myc that allows cap-independent translation. L-myc undergoes translation via the internal ribosome entry site and bypasses the typical eukaryotic cap-dependent translation pathway [1]. The myc family of genes when expressed are known to be involved in the control of cell growth, differentiation and apoptosis.

The Mnt internal ribosome entry site (IRES) is an RNA element. Mnt is a transcriptional repressor related to the Myc/Mad family of transcription factors. It is thought that this IRES allows efficient Mnt synthesis when cap-dependent translation initiation is reduced.

The N-myc internal ribosome entry site (IRES) is an RNA element found in the n-myc gene. The myc family of genes when expressed are known to be involved in the control of cell growth, differentiation and apoptosis. n-myc mRNA has an alternative method of translation via an internal ribosome entry site where ribosomes are recruited to the IRES located in the 5' UTR thus bypassing the typical eukaryotic cap-dependent translation pathway.
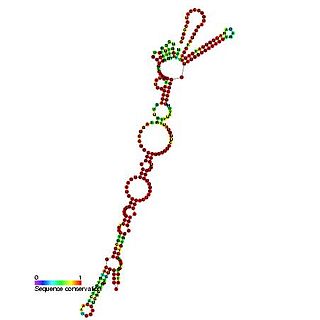
This family represents the Picornavirus internal ribosome entry site (IRES). IRES elements allow cap and end-independent translation of mRNA in the host cell. It has been found that La autoantigen (La) is required for Coxsackievirus B3 (CVB3) IRES-mediated translation, and it has been suggested that La may be required for the efficient translation of the viral RNA in the pancreas.

The HIF-1α internal ribosome entry site (IRES) is an RNA element present in the 5' UTR of the mRNA of HIF-1α that allows cap-independent translation. The HIF-1α internal ribosome entry site (IRES) allows translation to be maintained under hypoxic cell conditions that inhibit cap-dependent translation [1]. The hypoxia-inducible factor-1α protein (HIF-1α) is a subunit of the HIF-1 transcription factor, which induces transcription of several genes involved in the cellular response to hypoxia.
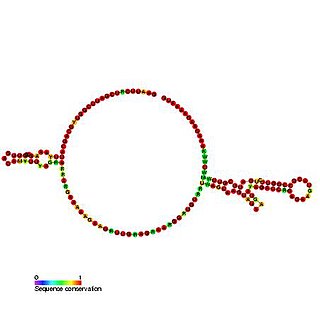
The Tobamovirus internal ribosome entry site (IRES) is an element that allows cap and end-independent translation of mRNA in the host cell. The IRES achieves this by mediating the internal initiation of translation by recruiting a ribosomal 43S pre-initiation complex directly to the initiation codon and eliminates the requirement for the eukaryotic initiation factor, eIF4F.
In molecular biology, the Ure2 internal ribosome entry site (IRES) is an RNA element present in the 5' UTR of the mRNA of Ure2. It allows 5' cap- and eIF4E-independent translation of an N-terminally truncated form of Ure2. This truncated form lacks the prion-forming domain. It is a 104 nucleotide region, smaller than most viral IRES elements, which forms a stem-loop structure. EIF2A represses this IRES resulting in an inhibition of translation of the N-terminally truncated Ure2.
In molecular biology, the ODC internal ribosome entry site (IRES) is an RNA element present in the 5' UTR of the mRNA encoding ornithine decarboxylase. It has been suggested that this IRES allows cap-independent translation of ornithine decarboxylase at the G2/M phase of the cell cycle, however there is some doubt about this. Translation from this IRES is activated by the zinc finger protein ZNF9 and by Poly(rC)-binding protein 2 (PCBP2). It is also activated in Ras-transformed cells.
















