
Pharmacogenomics is the study of the role of the genome in drug response. Its name reflects its combining of pharmacology and genomics. Pharmacogenomics analyzes how the genetic makeup of a patient affects their response to drugs. It deals with the influence of acquired and inherited genetic variation on drug response, by correlating DNA mutations with pharmacokinetic, pharmacodynamic, and/or immunogenic endpoints.

Cytochrome P450 3A4 is an important enzyme in the body, mainly found in the liver and in the intestine. It oxidizes small foreign organic molecules (xenobiotics), such as toxins or drugs, so that they can be removed from the body. It is highly homologous to CYP3A5, another important CYP3A enzyme.
Drug metabolism is the metabolic breakdown of drugs by living organisms, usually through specialized enzymatic systems. More generally, xenobiotic metabolism is the set of metabolic pathways that modify the chemical structure of xenobiotics, which are compounds foreign to an organism's normal biochemistry, such as any drug or poison. These pathways are a form of biotransformation present in all major groups of organisms and are considered to be of ancient origin. These reactions often act to detoxify poisonous compounds. The study of drug metabolism is called pharmacokinetics.

Nortriptyline, sold under the brand name Pamelor, among others, is a medication used to treat depression. This medicine is also sometimes used for neuropathic pain, attention deficit hyperactivity disorder (ADHD), smoking cessation and anxiety. As with many antidepressants, its use for young people with depression and other psychiatric disorders may be limited due to increased suicidality in the 18-24 population initiating treatment. Nortriptyline is a less preferred treatment for ADHD and stopping smoking. It is taken by mouth.
Acute intermittent porphyria (AIP) is a rare metabolic disorder affecting the production of heme resulting from a deficiency of the enzyme porphobilinogen deaminase. It is the most common of the acute porphyrias.

Thiopurine methyltransferase or thiopurine S-methyltransferase (TPMT) is an enzyme that in humans is encoded by the TPMT gene. A pseudogene for this locus is located on chromosome 18q.

Cytochrome P450 2C19 is an enzyme protein. It is a member of the CYP2C subfamily of the cytochrome P450 mixed-function oxidase system. This subfamily includes enzymes that catalyze metabolism of xenobiotics, including some proton pump inhibitors and antiepileptic drugs. In humans, it is the CYP2C19 gene that encodes the CYP2C19 protein. CYP2C19 is a liver enzyme that acts on at least 10% of drugs in current clinical use, most notably the antiplatelet treatment clopidogrel (Plavix), drugs that treat pain associated with ulcers, such as omeprazole, antiseizure drugs such as mephenytoin, the antimalarial proguanil, and the anxiolytic diazepam.

N-acetyltransferase (NAT) is an enzyme that catalyzes the transfer of acetyl groups from acetyl-CoA to arylamines, arylhydroxylamines and arylhydrazines. They have wide specificity for aromatic amines, particularly serotonin, and can also catalyze acetyl transfer between arylamines without CoA. N-acetyltransferases are cytosolic enzymes found in the liver and many tissues of most mammalian species, except the dog and fox, which cannot acetylate xenobiotics.
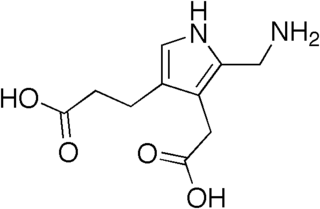
Porphobilinogen deaminase (hydroxymethylbilane synthase, or uroporphyrinogen I synthase) is an enzyme (EC 2.5.1.61) that in humans is encoded by the HMBS gene. Porphobilinogen deaminase is involved in the third step of the heme biosynthetic pathway. It catalyzes the head to tail condensation of four porphobilinogen molecules into the linear hydroxymethylbilane while releasing four ammonia molecules:
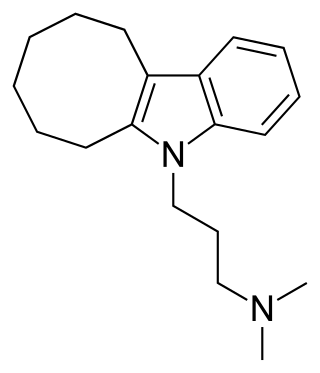
Iprindole, sold under the brand names Prondol, Galatur, and Tertran, is an atypical tricyclic antidepressant (TCA) that has been used in the United Kingdom and Ireland for the treatment of depression but appears to no longer be marketed. It was developed by Wyeth and was marketed in 1967. The drug has been described by some as the first "second-generation" antidepressant to be introduced. However, it was very little-used compared to other TCAs, with the number of prescriptions dispensed only in the thousands.

Fatty-acid amide hydrolase 1 or FAAH-1(EC 3.5.1.99, oleamide hydrolase, anandamide amidohydrolase) is a member of the serine hydrolase family of enzymes. It was first shown to break down anandamide (AEA) in 1993. In humans, it is encoded by the gene FAAH. FAAH also regulate the contents of N-acylethanolamine in Dictyostelium discoideum, as they modulate their levels in vivo through the use of a semispecific FAAH inhibitor.

Cytochrome P450 2B6 is an enzyme that in humans is encoded by the CYP2B6 gene. CYP2B6 is a member of the cytochrome P450 group of enzymes. Along with CYP2A6, it is involved with metabolizing nicotine, along with many other substances.

Aminolevulinic acid dehydratase (porphobilinogen synthase, or ALA dehydratase, or aminolevulinate dehydratase) is an enzyme (EC 4.2.1.24) that in humans is encoded by the ALAD gene. Porphobilinogen synthase (or ALA dehydratase, or aminolevulinate dehydratase) synthesizes porphobilinogen through the asymmetric condensation of two molecules of aminolevulinic acid. All natural tetrapyrroles, including hemes, chlorophylls and vitamin B12, share porphobilinogen as a common precursor. Porphobilinogen synthase is the prototype morpheein.

Solute carrier family 22 member 8, or organic anion transporter 3 (OAT3), is a protein that in humans is encoded by the SLC22A8 gene.

The Biozentrum of the University of Basel specializes in basic molecular and biomedical research and teaching. Research includes the areas of cell growth and development, infection biology, neurobiology, structural biology and biophysics, and computational and systems biology. With 550 employees, the Biozentrum is the largest department at the University of Basel's Faculty of Science. It is home to 32 research groups with scientists from more than 40 nations.
Christoph Handschin is a Swiss cell biologist at the Biozentrum University of Basel.

Markus Rüegg is a Swiss neurobiologist and professor at the Biozentrum of the University of Basel.

The flavin-containing monooxygenase (FMO) protein family specializes in the oxidation of xeno-substrates in order to facilitate the excretion of these compounds from living organisms. These enzymes can oxidize a wide array of heteroatoms, particularly soft nucleophiles, such as amines, sulfides, and phosphites. This reaction requires an oxygen, an NADPH cofactor, and an FAD prosthetic group. FMOs share several structural features, such as a NADPH binding domain, FAD binding domain, and a conserved arginine residue present in the active site. Recently, FMO enzymes have received a great deal of attention from the pharmaceutical industry both as a drug target for various diseases and as a means to metabolize pro-drug compounds into active pharmaceuticals. These monooxygenases are often misclassified because they share activity profiles similar to those of cytochrome P450 (CYP450), which is the major contributor to oxidative xenobiotic metabolism. However, a key difference between the two enzymes lies in how they proceed to oxidize their respective substrates; CYP enzymes make use of an oxygenated heme prosthetic group, while the FMO family utilizes FAD to oxidize its substrates.

Noroxycodone is the major metabolite of the opioid analgesic oxycodone. It is formed from oxycodone in the liver via N-demethylation predominantly by CYP3A4. Noroxycodone binds to and activates the μ-opioid receptor (MOR) similarly to oxycodone, although with one-third of the affinity of oxycodone and 5- to 10-fold lower activational potency. However, although a potent MOR agonist, noroxycodone poorly crosses the blood-brain-barrier into the central nervous system, and for this reason, is only minimally analgesic in comparison.

Matthias Schwab is a German doctor and university lecturer. He is director of the Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology located on the campus of the Robert-Bosch-Hospital in Stuttgart, an institution of the Robert Bosch Stiftung, and holder of the Chair of Clinical Pharmacology at the University of Tübingen as well as Medical Director of the Department of Clinical Pharmacology at the University Hospital Tübingen.



















