Related Research Articles
A hormone receptor is a receptor molecule that binds to a specific hormone. Hormone receptors are a wide family of proteins made up of receptors for thyroid and steroid hormones, retinoids and Vitamin D, and a variety of other receptors for various ligands, such as fatty acids and prostaglandins. Hormone receptors are of mainly two classes. Receptors for peptide hormones tend to be cell surface receptors built into the plasma membrane of cells and are thus referred to as trans membrane receptors. An example of this is Actrapid. Receptors for steroid hormones are usually found within the protoplasm and are referred to as intracellular or nuclear receptors, such as testosterone. Upon hormone binding, the receptor can initiate multiple signaling pathways, which ultimately leads to changes in the behavior of the target cells.
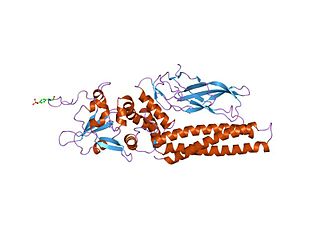
Members of the signal transducer and activator of transcription (STAT) protein family are intracellular transcription factors that mediate many aspects of cellular immunity, proliferation, apoptosis and differentiation. They are primarily activated by membrane receptor-associated Janus kinases (JAK). Dysregulation of this pathway is frequently observed in primary tumors and leads to increased angiogenesis which enhances the survival of tumors and immunosuppression. Gene knockout studies have provided evidence that STAT proteins are involved in the development and function of the immune system and play a role in maintaining immune tolerance and tumor surveillance.
The thyroid hormone receptor (TR) is a type of nuclear receptor that is activated by binding thyroid hormone. TRs act as transcription factors, ultimately affecting the regulation of gene transcription and translation. These receptors also have non-genomic effects that lead to second messenger activation, and corresponding cellular response.

The vitamin D receptor (VDR also known as the calcitriol receptor) is a member of the nuclear receptor family of transcription factors. Calcitriol (the active form of vitamin D, 1,25-(OH)2vitamin D3) binds to VDR, which then forms a heterodimer with the retinoid-X receptor. The VDR heterodimer then enters the nucleus and binds to Vitamin D responsive elements (VDRE) in genomic DNA. VDR binding results in expression or transrepression of many specific gene products. VDR is also involved in microRNA-directed post transcriptional mechanisms. In humans, the vitamin D receptor is encoded by the VDR gene located on chromosome 12q13.11.
The retinoic acid receptor (RAR) is a type of nuclear receptor which can also act as a ligand-activated transcription factor that is activated by both all-trans retinoic acid and 9-cis retinoic acid, retinoid active derivatives of Vitamin A. They are typically found within the nucleus. There are three retinoic acid receptors (RAR), RAR-alpha, RAR-beta, and RAR-gamma, encoded by the RARA, RARB, RARG genes, respectively. Within each RAR subtype there are various isoforms differing in their N-terminal region A. Multiple splice variants have been identified in human RARs: four for RARA, five for RARB, and two for RARG. As with other type II nuclear receptors, RAR heterodimerizes with RXR and in the absence of ligand, the RAR/RXR dimer binds to hormone response elements known as retinoic acid response elements (RAREs) complexed with corepressor protein. Binding of agonist ligands to RAR results in dissociation of corepressor and recruitment of coactivator protein that, in turn, promotes transcription of the downstream target gene into mRNA and eventually protein. In addition, the expression of RAR genes is under epigenetic regulation by promoter methylation. Both the length and magnitude of the retinoid response is dependent of the degradation of RARs and RXRs through the ubiquitin-proteasome. This degradation can lead to elongation of the DNA transcription through disruption of the initiation complex or to end the response to facilitate further transcriptional programs. RAR receptors are also known to exhibit many retinoid-independent effects as they bind to and regulate other nuclear receptor pathways, such as the estrogen receptor.
The retinoid X receptor (RXR) is a type of nuclear receptor that is activated by 9-cis retinoic acid, which is discussed controversially to be of endogenous relevance, and 9-cis-13,14-dihydroretinoic acid, which may be an endogenous mammalian RXR-selective agonist. Bexarotene is the only specific activator of the RXRs which does not activate the Retinoic Acid Receptors.

The liver X receptor (LXR) is a member of the nuclear receptor family of transcription factors and is closely related to nuclear receptors such as the PPARs, FXR and RXR. Liver X receptors (LXRs) are important regulators of cholesterol, fatty acid, and glucose homeostasis. LXRs were earlier classified as orphan nuclear receptors, however, upon discovery of endogenous oxysterols as ligands they were subsequently deorphanized.
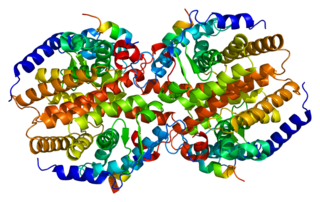
The constitutive androstane receptor (CAR) also known as nuclear receptor subfamily 1, group I, member 3 is a protein that in humans is encoded by the NR1I3 gene. CAR is a member of the nuclear receptor superfamily and along with pregnane X receptor (PXR) functions as a sensor of endobiotic and xenobiotic substances. In response, expression of proteins responsible for the metabolism and excretion of these substances is upregulated. Hence, CAR and PXR play a major role in the detoxification of foreign substances such as drugs.
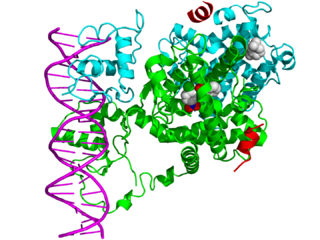
In the field of molecular biology, nuclear receptors are a class of proteins responsible for sensing steroids, thyroid hormones, vitamins, and certain other molecules. These intracellular receptors work with other proteins to regulate the expression of specific genes, thereby controlling the development, homeostasis, and metabolism of the organism.

The nuclear receptor 4A1 also known as Nur77, TR3, and NGFI-B is a protein that in humans is encoded by the NR4A1 gene.

The small heterodimer partner (SHP) also known as NR0B2 is a protein that in humans is encoded by the NR0B2 gene. SHP is a member of the nuclear receptor family of intracellular transcription factors. SHP is unusual for a nuclear receptor in that it lacks a DNA binding domain. Therefore, it is technically neither a transcription factor nor nuclear receptor but nevertheless it is still classified as such due to relatively high sequence homology with other nuclear receptor family members.

Retinoid X receptor alpha (RXR-alpha), also known as NR2B1 is a nuclear receptor that in humans is encoded by the RXRA gene.

Retinoic acid receptor alpha (RAR-α), also known as NR1B1, is a nuclear receptor that in humans is encoded by the RARA gene.

Retinoid X receptor gamma (RXR-gamma), also known as NR2B3 is a nuclear receptor that in humans is encoded by the RXRG gene.

Retinoid X receptor beta (RXR-beta), also known as NR2B2 is a nuclear receptor that in humans is encoded by the RXRB gene.
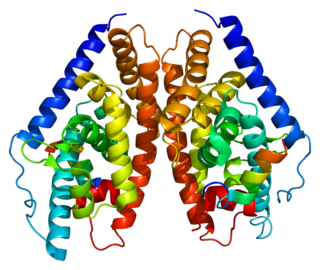
Liver X receptor beta (LXR-β) is a member of the nuclear receptor family of transcription factors. LXR-β is encoded by the NR1H2 gene.
Response elements are short sequences of DNA within a gene promoter or enhancer region that are able to bind specific transcription factors and regulate transcription of genes.

Insulin induced gene 2, also known as INSIG2, is a protein which in humans is encoded by the INSIG2 gene.
The ecdysone receptor is a nuclear receptor found in arthropods, where it controls development and contributes to other processes such as reproduction. The receptor is a non-covalent heterodimer of two proteins, the EcR protein and ultraspiracle protein (USP). It binds to and is activated by ecdysteroids. Insect ecdysone receptors are currently better characterized than those from other arthropods, and mimics of ecdysteroids are used commercially as caterpillar-selective insecticides.
Vitamin D is a group of fat-soluble prohormones.
References
- ↑ Roff A, Wilson RT (March 2009). "A Novel SNP in a Vitamin D Response Element of the CYP24A1 Promoter Reduces Protein Binding, Transactivation, and Gene Expression". J Steroid Biochem Mol Biol . 112 (1–3): 47–54. doi:10.1016/j.jsbmb.2008.08.009. PMC 2749287 . PMID 18824104.
- ↑ Jin CH, Pike JW (1996). "Human vitamin D receptor-dependent transactivation in Saccharomyces cerevisiae requires retinoid X receptor". Mol. Endocrinol. 10 (2): 196–205. doi: 10.1210/mend.10.2.8825559 . PMID 8825559.
- ↑ Umesono K, Murakami KK, Thompson CC, Evans RM (June 1991). "Direct repeats as selective response elements for the thyroid hormone, retinoic acid, and vitamin D3 receptors". Cell . 65 (7): 1255–66. doi:10.1016/0092-8674(91)90020-Y. PMC 6159884 . PMID 1648450.
- ↑ De Groot LJ, Beck-Peccoz P, Chrousos G, Dungan K, Grossman A, Hershman JM, Koch C, McLachlan R, New M, Rebar R, Singer F, Vinik A, Weickert MO, Bikle D (2014). "Vitamin D: Production, Metabolism, and Mechanisms of Action". Endotext [Internet]. South Dartmouth (MA): MDText.com. PMID 25905172.