
Phospholipids are a class of lipids whose molecule has a hydrophilic "head" containing a phosphate group and two hydrophobic "tails" derived from fatty acids, joined by an alcohol residue. Marine phospholipids typically have omega-3 fatty acids EPA and DHA integrated as part of the phospholipid molecule. The phosphate group can be modified with simple organic molecules such as choline, ethanolamine or serine.

In cell biology, a vesicle is a structure within or outside a cell, consisting of liquid or cytoplasm enclosed by a lipid bilayer. Vesicles form naturally during the processes of secretion (exocytosis), uptake (endocytosis) and transport of materials within the plasma membrane. Alternatively, they may be prepared artificially, in which case they are called liposomes. If there is only one phospholipid bilayer, the vesicles are called unilamellar liposomes; otherwise they are called multilamellar liposomes. The membrane enclosing the vesicle is also a lamellar phase, similar to that of the plasma membrane, and intracellular vesicles can fuse with the plasma membrane to release their contents outside the cell. Vesicles can also fuse with other organelles within the cell. A vesicle released from the cell is known as an extracellular vesicle.

The lipid bilayer is a thin polar membrane made of two layers of lipid molecules. These membranes are flat sheets that form a continuous barrier around all cells. The cell membranes of almost all organisms and many viruses are made of a lipid bilayer, as are the nuclear membrane surrounding the cell nucleus, and membranes of the membrane-bound organelles in the cell. The lipid bilayer is the barrier that keeps ions, proteins and other molecules where they are needed and prevents them from diffusing into areas where they should not be. Lipid bilayers are ideally suited to this role, even though they are only a few nanometers in width, because they are impermeable to most water-soluble (hydrophilic) molecules. Bilayers are particularly impermeable to ions, which allows cells to regulate salt concentrations and pH by transporting ions across their membranes using proteins called ion pumps.

A liposome is a small artificial vesicle, spherical in shape, having at least one lipid bilayer. Due to their hydrophobicity and/or hydrophilicity, biocompatibility, particle size and many other properties, liposomes can be used as drug delivery vehicles for administration of pharmaceutical drugs and nutrients, such as lipid nanoparticles in mRNA vaccines, and DNA vaccines. Liposomes can be prepared by disrupting biological membranes.

Dipalmitoylphosphatidylcholine (DPPC) is a phospholipid (and a lecithin) consisting of two C16 palmitic acid groups attached to a phosphatidylcholine head-group.
An artificial cell, synthetic cell or minimal cell is an engineered particle that mimics one or many functions of a biological cell. Often, artificial cells are biological or polymeric membranes which enclose biologically active materials. As such, liposomes, polymersomes, nanoparticles, microcapsules and a number of other particles can qualify as artificial cells.

A virosome is a drug or vaccine delivery mechanism consisting of unilamellar phospholipid membrane vesicle incorporating virus derived proteins to allow the virosomes to fuse with target cells. Viruses are infectious agents that can replicate in their host organism, however virosomes do not replicate. The properties that virosomes share with viruses are based on their structure; virosomes are essentially safely modified viral envelopes that contain the phospholipid membrane and surface glycoproteins. As a drug or vaccine delivery mechanism they are biologically compatible with many host organisms and are also biodegradable. The use of reconstituted virally derived proteins in the formation of the virosome allows for the utilization of what would otherwise be the immunogenic properties of a live-attenuated virus, but is instead a safely killed virus. A safely killed virus can serve as a promising vector because it won't cause infection and the viral structure allows the virosome to recognize specific components of its target cells.

Cationic liposomes are spherical structures that contain positively charged lipids. Cationic liposomes can vary in size between 40 nm and 500 nm, and they can either have one lipid bilayer (monolamellar) or multiple lipid bilayers (multilamellar). The positive charge of the phospholipids allows cationic liposomes to form complexes with negatively charged nucleic acids through ionic interactions. Upon interacting with nucleic acids, cationic liposomes form clusters of aggregated vesicles. These interactions allow cationic liposomes to condense and encapsulate various therapeutic and diagnostic agents in their aqueous compartment or in their lipid bilayer. These cationic liposome-nucleic acid complexes are also referred to as lipoplexes. Due to the overall positive charge of cationic liposomes, they interact with negatively charged cell membranes more readily than classic liposomes. This positive charge can also create some issues in vivo, such as binding to plasma proteins in the bloodstream, which leads to opsonization. These issues can be reduced by optimizing the physical and chemical properties of cationic liposomes through their lipid composition. Cationic liposomes are increasingly being researched for use as delivery vectors in gene therapy due to their capability to efficiently transfect cells. A common application for cationic liposomes is cancer drug delivery.

Polymorphism in biophysics is the ability of lipids to aggregate in a variety of ways, giving rise to structures of different shapes, known as "phases". This can be in the form of sphere of lipid molecules (micelles), pairs of layers that face one another, a tubular arrangement (hexagonal), or various cubic phases. More complicated aggregations have also been observed, such as rhombohedral, tetragonal and orthorhombic phases.
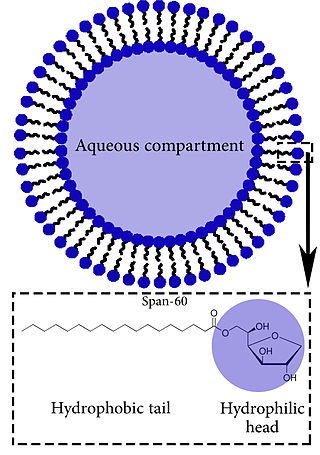
Niosomes are utilized for drug delivery to specific sites in order to achieve desired therapeutic effects. They provide an alternative to liposomes and are composed of non-ionic surfactant-based vesicles that include non-ionic surfactant and cholesterol as an excipient, which can potentially enhance drug absorption. Structurally, niosomes are similar to liposomes, as they both consist of a lipid bilayer. However, niosomes are more stable during the formation process and storage than liposomes. They can trap hydrophilic and lipophilic drugs, either in an aqueous layer or in a vesicular membrane composed of lipid material.

In membrane biology, fusion is the process by which two initially distinct lipid bilayers merge their hydrophobic cores, resulting in one interconnected structure. If this fusion proceeds completely through both leaflets of both bilayers, an aqueous bridge is formed and the internal contents of the two structures can mix. Alternatively, if only one leaflet from each bilayer is involved in the fusion process, the bilayers are said to be hemifused. In hemifusion, the lipid constituents of the outer leaflet of the two bilayers can mix, but the inner leaflets remain distinct. The aqueous contents enclosed by each bilayer also remain separated.
A model lipid bilayer is any bilayer assembled in vitro, as opposed to the bilayer of natural cell membranes or covering various sub-cellular structures like the nucleus. They are used to study the fundamental properties of biological membranes in a simplified and well-controlled environment, and increasingly in bottom-up synthetic biology for the construction of artificial cells. A model bilayer can be made with either synthetic or natural lipids. The simplest model systems contain only a single pure synthetic lipid. More physiologically relevant model bilayers can be made with mixtures of several synthetic or natural lipids.
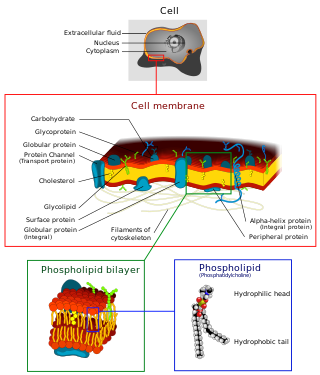
The cell membrane is a biological membrane that separates and protects the interior of all cells from the outside environment. The cell membrane consists of a lipid bilayer, made up of two layers of phospholipids with cholesterols interspersed between them, maintaining appropriate membrane fluidity at various temperatures. The membrane also contains membrane proteins, including integral proteins that span the membrane and serve as membrane transporters, and peripheral proteins that loosely attach to the outer (peripheral) side of the cell membrane, acting as enzymes to facilitate interaction with the cell's environment. Glycolipids embedded in the outer lipid layer serve a similar purpose. The cell membrane controls the movement of substances in and out of cells and organelles, being selectively permeable to ions and organic molecules. In addition, cell membranes are involved in a variety of cellular processes such as cell adhesion, ion conductivity, and cell signalling and serve as the attachment surface for several extracellular structures, including the cell wall and the carbohydrate layer called the glycocalyx, as well as the intracellular network of protein fibers called the cytoskeleton. In the field of synthetic biology, cell membranes can be artificially reassembled.
Nanoparticles for drug delivery to the brain is a method for transporting drug molecules across the blood–brain barrier (BBB) using nanoparticles. These drugs cross the BBB and deliver pharmaceuticals to the brain for therapeutic treatment of neurological disorders. These disorders include Parkinson's disease, Alzheimer's disease, schizophrenia, depression, and brain tumors. Part of the difficulty in finding cures for these central nervous system (CNS) disorders is that there is yet no truly efficient delivery method for drugs to cross the BBB. Antibiotics, antineoplastic agents, and a variety of CNS-active drugs, especially neuropeptides, are a few examples of molecules that cannot pass the BBB alone. With the aid of nanoparticle delivery systems, however, studies have shown that some drugs can now cross the BBB, and even exhibit lower toxicity and decrease adverse effects throughout the body. Toxicity is an important concept for pharmacology because high toxicity levels in the body could be detrimental to the patient by affecting other organs and disrupting their function. Further, the BBB is not the only physiological barrier for drug delivery to the brain. Other biological factors influence how drugs are transported throughout the body and how they target specific locations for action. Some of these pathophysiological factors include blood flow alterations, edema and increased intracranial pressure, metabolic perturbations, and altered gene expression and protein synthesis. Though there exist many obstacles that make developing a robust delivery system difficult, nanoparticles provide a promising mechanism for drug transport to the CNS.
A unilamellar liposome is a spherical liposome, a vesicle, bounded by a single bilayer of an amphiphilic lipid or a mixture of such lipids, containing aqueous solution inside the chamber. Unilamellar liposomes are used to study biological systems and to mimic cell membranes, and are classified into three groups based on their size: small unilamellar liposomes/vesicles (SUVs) that with a size range of 20–100 nm, large unilamellar liposomes/vesicles (LUVs) with a size range of 100–1000 nm and giant unilamellar liposomes/vesicles (GUVs) with a size range of 1-200 µm. GUVs are mostly used as models for biological membranes in research work. Animal cells are 10–30 µm and plant cells are typically 10–100 µm. Even smaller cell organelles such as mitochondria are typically 1-2 µm. Therefore, a proper model should account for the size of the specimen being studied. In addition, the size of vesicles dictates their membrane curvature which is an important factor in studying fusion proteins. SUVs have a higher membrane curvature and vesicles with high membrane curvature can promote membrane fusion faster than vesicles with lower membrane curvature such as GUVs.

Lamellar phase refers generally to packing of polar-headed long chain nonpolar-tail molecules in an environment of bulk polar liquid, as sheets of bilayers separated by bulk liquid. In biophysics, polar lipids pack as a liquid crystalline bilayer, with hydrophobic fatty acyl long chains directed inwardly and polar headgroups of lipids aligned on the outside in contact with water, as a 2-dimensional flat sheet surface. Under transmission electron microscope (TEM), after staining with polar headgroup reactive chemical osmium tetroxide, lamellar lipid phase appears as two thin parallel dark staining lines/sheets, constituted by aligned polar headgroups of lipids. 'Sandwiched' between these two parallel lines, there exists one thicker line/sheet of non-staining closely packed layer of long lipid fatty acyl chains. This TEM-appearance became famous as Robertson's unit membrane - the basis of all biological membranes, and structure of lipid bilayer in unilamellar liposomes. In multilamellar liposomes, many such lipid bilayer sheets are layered concentrically with water layers in between.
Cubosomes are discrete, sub-micron, nanostructured particles of the bicontinuous cubic liquid crystalline phase. The term "bicontinuous" refers to two distinct hydrophilic regions separated by the bilayer. Bicontinuous cubic crystalline materials have been an active research topic because their structure lends itself well to controlled-release applications.
Joseph Anthony Zasadzinski, also known as "Joe Z" is an American chemical engineer from the University of California, Santa Barbara. He was awarded the status of Fellow in the American Physical Society, after he was nominated by his Division of Biological Physics in 2008, for "applying physical principles of self-assembly, directed assembly and bio-mimicry to create well-controlled lipid structures such as unilamellar vesicles and "vesosomes" for biomedical applications such as targeted drug-delivery vehicles and treatments for respiratory diseases, and for developing new microscopies." Zasadzinski currently works in the Department of Chemical Engineering and Materials Science at the University of Minnesota, Twin Cities.

Ethosomes are phospholipid nanovesicles used for dermal and transdermal delivery of molecules. Ethosomes were developed by Touitou et al.,1997, as additional novel lipid carriers composed of ethanol, phospholipids, and water. They are reported to improve the skin delivery of various drugs. Ethanol is an efficient permeation enhancer that is believed to act by affecting the intercellular region of the stratum corneum. Ethosomes are soft malleable vesicles composed mainly of phospholipids, ethanol, and water. These soft vesicles represent novel vesicles carriers for enhanced delivery through the skin. The size of the ethosomes vesicles can be modulated from tens of nanometers to microns.

A liposome extruder is a device that prepares cell membranes, exosomes and also generates nanoscale liposome formulations. The liposome extruder employs the track-etched membrane to filter huge particles and achieve sterile filtration.












