Related Research Articles
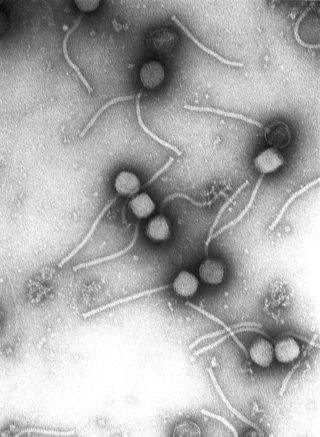
Virology is the scientific study of biological viruses. It is a subfield of microbiology that focuses on their detection, structure, classification and evolution, their methods of infection and exploitation of host cells for reproduction, their interaction with host organism physiology and immunity, the diseases they cause, the techniques to isolate and culture them, and their use in research and therapy.
In vitro toxicity testing is the scientific analysis of the toxic effects of chemical substances on cultured bacteria or mammalian cells. In vitro testing methods are employed primarily to identify potentially hazardous chemicals and/or to confirm the lack of certain toxic properties in the early stages of the development of potentially useful new substances such as therapeutic drugs, agricultural chemicals and food additives.
Cytotoxicity is the quality of being toxic to cells. Examples of toxic agents are toxic metals, toxic chemicals, microbe neurotoxins, radiation particles and even specific neurotransmitters when the system is out of balance. Also some types of venom, e.g. from the puff adder or brown recluse spider are toxic to cells.
An assay is an investigative (analytic) procedure in laboratory medicine, mining, pharmacology, environmental biology and molecular biology for qualitatively assessing or quantitatively measuring the presence, amount, or functional activity of a target entity. The measured entity is often called the analyte, the measurand, or the target of the assay. The analyte can be a drug, biochemical substance, chemical element or compound, or cell in an organism or organic sample. An assay usually aims to measure an analyte's intensive property and express it in the relevant measurement unit.

Bacteriological water analysis is a method of analysing water to estimate the numbers of bacteria present and, if needed, to find out what sort of bacteria they are. It represents one aspect of water quality. It is a microbiological analytical procedure which uses samples of water and from these samples determines the concentration of bacteria. It is then possible to draw inferences about the suitability of the water for use from these concentrations. This process is used, for example, to routinely confirm that water is safe for human consumption or that bathing and recreational waters are safe to use.

A viral plaque is a visible structure formed after introducing a viral sample to a cell culture grown on some nutrient medium. The virus will replicate and spread, generating regions of cell destruction known as plaques. For example, Vero cell or other tissue cultures may be used to investigate an influenza virus or coronavirus, while various bacterial cultures would be used for bacteriophages.
The hemagglutination assay or haemagglutination assay (HA) and the hemagglutination inhibition assay were developed in 1941–42 by American virologist George Hirst as methods for quantifying the relative concentration of viruses, bacteria, or antibodies.
Hemagglutination, or haemagglutination, is a specific form of agglutination that involves red blood cells (RBCs). It has two common uses in the laboratory: blood typing and the quantification of virus dilutions in a haemagglutination assay.

Neutral red is a eurhodin dye used for staining in histology. It stains lysosomes red. It is used as a general stain in histology, as a counterstain in combination with other dyes, and for many staining methods. Together with Janus Green B, it is used to stain embryonal tissues and supravital staining of blood. Can be used for staining Golgi apparatus in cells and Nissl granules in neurons.
In microbiology, colony-forming unit is a unit which estimates the number of microbial cells in a sample that are viable, able to multiply via binary fission under the controlled conditions. Counting with colony-forming units requires culturing the microbes and counts only viable cells, in contrast with microscopic examination which counts all cells, living or dead. The visual appearance of a colony in a cell culture requires significant growth, and when counting colonies, it is uncertain if the colony arose from one cell or a group of cells. Expressing results as colony-forming units reflects this uncertainty.
Dilution cloning or cloning by limiting dilution describes a procedure to obtain a monoclonal cell population starting from a polyclonal mass of cells. This is achieved by setting up a series of increasing dilutions of the parent (polyclonal) cell culture. A suspension of the parent cells is made. Appropriate dilutions are then made, depending on cell number in the starting population, as well as the viability and characteristics of the cells being cloned. After the final dilutions are produced, aliquots of the suspension are plated or placed in wells and incubated. If all works correctly, a monoclonal cell colony will be produced. Applications for the procedure include cloning of parasites, T cells, transgenic cells, macrophages. and hematopoietic stem cells.

Phloxine B is a water-soluble red dye used for coloring drugs and cosmetics in the United States and coloring food in Japan. It is derived from fluorescein, but differs by the presence of four bromine atoms at positions 2, 4, 5 and 7 of the xanthene ring and four chlorine atoms in the carboxyphenyl ring. It has an absorption maximum around 540 nm and an emission maximum around 564 nm. Apart from industrial use, phloxine B has functions as an antimicrobial substance, viability dye and biological stain. For example, it is used in hematoxylin-phloxine-saffron (HPS) staining to color the cytoplasm and connective tissue in shades of red.
The most probable number method, otherwise known as the method of Poisson zeroes, is a method of getting quantitative data on concentrations of discrete items from positive/negative (incidence) data.
CASY technology is an electric field multi-channel cell counting system. It was first marketed by Schärfe System GmbH in 1987 under the name CASY1. The first systems were sold with an ATARI computer and a rectangular chassis. In the 1990s the ATARI computer got replaced by a common PC and the chassis changed into cylinders. In 2006, Schärfe System was acquired by Innovatis AG, a company focused on cell culture analysis. CASY utilizes the techniques of electric current exclusion and pulse area analysis, the cells can be analyzed and counted in an efficient and precise manner. This technology can be applied for cell counting, cell culture analysis at a certain time interval, or even a period of time.
Virus quantification is counting or calculating the number of virus particles (virions) in a sample to determine the virus concentration. It is used in both research and development (R&D) in academic and commercial laboratories as well as in production situations where the quantity of virus at various steps is an important variable that must be monitored. For example, the production of virus-based vaccines, recombinant proteins using viral vectors, and viral antigens all require virus quantification to continually monitor and/or modify the process in order to optimize product quality and production yields and to respond to ever changing demands and applications. Other examples of specific instances where viruses need to be quantified include clone screening, multiplicity of infection (MOI) optimization, and adaptation of methods to cell culture.

A viability assay is an assay that is created to determine the ability of organs, cells or tissues to maintain or recover a state of survival. Viability can be distinguished from the all-or-nothing states of life and death by the use of a quantifiable index that ranges between the integers of 0 and 1 or, if more easily understood, the range of 0% and 100%. Viability can be observed through the physical properties of cells, tissues, and organs. Some of these include mechanical activity, motility, such as with spermatozoa and granulocytes, the contraction of muscle tissue or cells, mitotic activity in cellular functions, and more. Viability assays provide a more precise basis for measurement of an organism's level of vitality.
Cell counting is any of various methods for the counting or similar quantification of cells in the life sciences, including medical diagnosis and treatment. It is an important subset of cytometry, with applications in research and clinical practice. For example, the complete blood count can help a physician to determine why a patient feels unwell and what to do to help. Cell counts within liquid media are usually expressed as a number of cells per unit of volume, thus expressing a concentration.
Virtual colony count (VCC) is a kinetic, 96-well microbiological assay originally developed to measure the activity of defensins. It has since been applied to other antimicrobial peptides including LL-37. It utilizes a method of enumerating bacteria called quantitative growth kinetics, which compares the time taken for a bacterial batch culture to reach a threshold optical density with that of a series of calibration curves. The name VCC has also been used to describe the application of quantitative growth kinetics to enumerate bacteria in cell culture infection models. Antimicrobial susceptibility testing (AST) can be done on 96-well plates by diluting the antimicrobial agent at varying concentrations in broth inoculated with bacteria and measuring the minimum inhibitory concentration that results in no growth. However, these methods cannot be used to study some membrane-active antimicrobial peptides, which are inhibited by the broth itself. The virtual colony count procedure takes advantage of this fact by first exposing bacterial cells to the active antimicrobial agent in a low-salt buffer for two hours, then simultaneously inhibiting antimicrobial activity and inducing exponential growth by adding broth. The growth kinetics of surviving cells can then be monitored using a temperature-controlled plate reader. The time taken for each growth curve to reach a threshold change in optical density is then converted into virtual survival values, which serve as a measure of antimicrobial activity.

Indirect immunoperoxidase assay (IPA) is a laboratory technique used to detect and titrate viruses that do not cause measurable cytopathic effects and cannot be measured by classical plaque assays. These viruses include human coronavirus 229E and OC43.
In microbiology, the term isolation refers to the separation of a strain from a natural, mixed population of living microbes, as present in the environment, for example in water or soil, or from living beings with skin flora, oral flora or gut flora, in order to identify the microbe(s) of interest. Historically, the laboratory techniques of isolation first developed in the field of bacteriology and parasitology, before those in virology during the 20th century.
References
- ↑ "Invitrogen viable cell count method" (PDF). Retrieved 4 June 2013.
- ↑ "Plaque assay protocol" (PDF). Retrieved 4 June 2013.