Related Research Articles

Bacterial growth is proliferation of bacterium into two daughter cells, in a process called binary fission. Providing no event occurs, the resulting daughter cells are genetically identical to the original cell. Hence, bacterial growth occurs. Both daughter cells from the division do not necessarily survive. However, if the surviving number exceeds unity on average, the bacterial population undergoes exponential growth. The measurement of an exponential bacterial growth curve in batch culture was traditionally a part of the training of all microbiologists; the basic means requires bacterial enumeration by direct and individual, direct and bulk (biomass), indirect and individual, or indirect and bulk methods. Models reconcile theory with the measurements.

The human microbiome is the aggregate of all microbiota that reside on or within human tissues and biofluids along with the corresponding anatomical sites in which they reside, including the skin, mammary glands, seminal fluid, uterus, ovarian follicles, lung, saliva, oral mucosa, conjunctiva, biliary tract, and gastrointestinal tract. Types of human microbiota include bacteria, archaea, fungi, protists and viruses. Though micro-animals can also live on the human body, they are typically excluded from this definition. In the context of genomics, the term human microbiome is sometimes used to refer to the collective genomes of resident microorganisms; however, the term human metagenome has the same meaning.

An agar plate is a Petri dish that contains a growth medium solidified with agar, used to culture microorganisms. Sometimes selective compounds are added to influence growth, such as antibiotics.
An antimicrobial is an agent that kills microorganisms or stops their growth. Antimicrobial medicines can be grouped according to the microorganisms they act primarily against. For example, antibiotics are used against bacteria, and antifungals are used against fungi. They can also be classified according to their function. Agents that kill microbes are microbicides, while those that merely inhibit their growth are called bacteriostatic agents. The use of antimicrobial medicines to treat infection is known as antimicrobial chemotherapy, while the use of antimicrobial medicines to prevent infection is known as antimicrobial prophylaxis.

A blood culture is a medical laboratory test used to detect bacteria or fungi in a person's blood. Under normal conditions, the blood does not contain microorganisms: their presence can indicate a bloodstream infection such as bacteremia or fungemia, which in severe cases may result in sepsis. By culturing the blood, microbes can be identified and tested for resistance to antimicrobial drugs, which allows clinicians to provide an effective treatment.

A growth medium or culture medium is a solid, liquid, or semi-solid designed to support the growth of a population of microorganisms or cells via the process of cell proliferation or small plants like the moss Physcomitrella patens. Different types of media are used for growing different types of cells.
Osmotrophy is a feeding mechanism involving the movement of dissolved organic compounds by osmosis for nutrition. Organisms that use osmotrophy are called osmotrophs. Some mixotrophic microorganisms use osmotrophy to derive some of their energy. Osmotrophy is used by a diversity of organisms. Organisms that use osmotrophy include bacteria, many species of protists and most fungi. Some macroscopic animals like molluscs, sponges, corals, brachiopods and echinoderms may use osmotrophic feeding as a supplemental food source.
Industrial fermentation is the intentional use of fermentation in manufacturing products useful to humans. In addition to the mass production of fermented foods and drinks, industrial fermentation has widespread applications in chemical industry. Commodity chemicals, such as acetic acid, citric acid, and ethanol are made by fermentation. Moreover, nearly all commercially produced industrial enzymes, such as lipase, invertase and rennet, are made by fermentation with genetically modified microbes. In some cases, production of biomass itself is the objective, as is the case for single-cell proteins, baker's yeast, and starter cultures for lactic acid bacteria used in cheesemaking.
Trypsinization is the process of cell dissociation using trypsin, a proteolytic enzyme which breaks down proteins, to dissociate adherent cells from the vessel in which they are being cultured. When added to a cell culture, trypsin breaks down the proteins that enable the cells to adhere to the vessel. Trypsinization is often used to pass cells to a new vessel. When the trypsinization process is complete the cells will be in suspension and appear rounded.

A chemostat is a bioreactor to which fresh medium is continuously added, while culture liquid containing left over nutrients, metabolic end products and microorganisms is continuously removed at the same rate to keep the culture volume constant. By changing the rate with which medium is added to the bioreactor the specific growth rate of the microorganism can be easily controlled within limits.
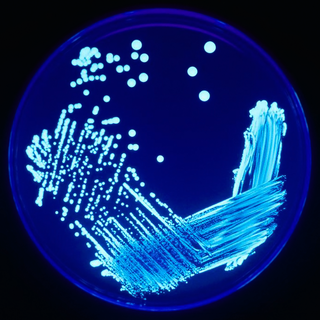
In microbiology, streaking is a technique used to isolate a pure strain from a single species of microorganism, often bacteria. Samples can then be taken from the resulting colonies and a microbiological culture can be grown on a new plate so that the organism can be identified, studied, or tested.
Fed-batch culture is, in the broadest sense, defined as an operational technique in biotechnological processes where one or more nutrients (substrates) are fed (supplied) to the bioreactor during cultivation and in which the product(s) remain in the bioreactor until the end of the run. An alternative description of the method is that of a culture in which "a base medium supports initial cell culture and a feed medium is added to prevent nutrient depletion". It is also a type of semi-batch culture. In some cases, all the nutrients are fed into the bioreactor. The advantage of the fed-batch culture is that one can control concentration of fed-substrate in the culture liquid at arbitrarily desired levels.

Phototrophic biofilms are microbial communities generally comprising both phototrophic microorganisms, which use light as their energy source, and chemoheterotrophs. Thick laminated multilayered phototrophic biofilms are usually referred to as microbial mats or phototrophic mats. These organisms, which can be prokaryotic or eukaryotic organisms like bacteria, cyanobacteria, fungi, and microalgae, make up diverse microbial communities that are affixed in a mucous matrix, or film. These biofilms occur on contact surfaces in a range of terrestrial and aquatic environments. The formation of biofilms is a complex process and is dependent upon the availability of light as well as the relationships between the microorganisms. Biofilms serve a variety of roles in aquatic, terrestrial, and extreme environments; these roles include functions which are both beneficial and detrimental to the environment. In addition to these natural roles, phototrophic biofilms have also been adapted for applications such as crop production and protection, bioremediation, and wastewater treatment.

Biopreservation is the use of natural or controlled microbiota or antimicrobials as a way of preserving food and extending its shelf life. The biopreservation of food, especially utilizing lactic acid bacteria (LAB) that are inhibitory to food spoilage microbes, has been practiced since early ages, at first unconsciously but eventually with an increasingly robust scientific foundation. Beneficial bacteria or the fermentation products produced by these bacteria are used in biopreservation to control spoilage and render pathogens inactive in food. There are a various modes of action through which microorganisms can interfere with the growth of others such as organic acid production, resulting in a reduction of pH and the antimicrobial activity of the un-dissociated acid molecules, a wide variety of small inhibitory molecules including hydrogen peroxide, etc. It is a benign ecological approach which is gaining increasing attention.
Soil microbiology is the study of microorganisms in soil, their functions, and how they affect soil properties. It is believed that between two and four billion years ago, the first ancient bacteria and microorganisms came about on Earth's oceans. These bacteria could fix nitrogen, in time multiplied, and as a result released oxygen into the atmosphere. This led to more advanced microorganisms, which are important because they affect soil structure and fertility. Soil microorganisms can be classified as bacteria, actinomycetes, fungi, algae and protozoa. Each of these groups has characteristics that define them and their functions in soil.
Microbial enhanced oil recovery (MEOR) is a biological based technology consisting in manipulating function or structure, or both, of microbial environments existing in oil reservoirs. The ultimate aim of MEOR is to improve the recovery of oil entrapped in porous media while increasing economic profits. MEOR is a tertiary oil extraction technology allowing the partial recovery of the commonly residual two-thirds of oil, thus extending the life of mature oil reservoirs.
In microbiology, the term isolation refers to the separation of a strain from a natural, mixed population of living microbes, as present in the environment, for example in water or soil, or from living beings with skin flora, oral flora or gut flora, in order to identify the microbe(s) of interest. Historically, the laboratory techniques of isolation first developed in the field of bacteriology and parasitology, before those in virology during the 20th century.
Diagnostic microbiology is the study of microbial identification. Since the discovery of the germ theory of disease, scientists have been finding ways to harvest specific organisms. Using methods such as differential media or genome sequencing, physicians and scientists can observe novel functions in organisms for more effective and accurate diagnosis of organisms. Methods used in diagnostic microbiology are often used to take advantage of a particular difference in organisms and attain information about what species it can be identified as, which is often through a reference of previous studies. New studies provide information that others can reference so that scientists can attain a basic understanding of the organism they are examining.

A cell suspension or suspension culture is a type of cell culture in which single cells or small aggregates of cells are allowed to function and multiply in an agitated growth medium, thus forming a suspension. Suspension culture is one of the two classical types of cell culture, the other being adherent culture. The history of suspension cell culture closely aligns with the history of cell culture overall, but differs in maintenance methods and commercial applications. The cells themselves can either be derived from homogenized tissue or from heterogenous cell solutions. Suspension cell culture is commonly used to culture nonadhesive cell lines like hematopoietic cells, plant cells, and insect cells. While some cell lines are cultured in suspension, the majority of commercially available mammalian cell lines are adherent. Suspension cell cultures must be agitated to maintain cells in suspension, and may require specialized equipment and flasks. These cultures need to be maintained with nutrient containing media and cultured in a specific cell density range to avoid cell death.
Entomoculture is the subfield of cellular agriculture which specifically deals with the production of insect tissue in vitro. It draws on principles more generally used in tissue engineering and has scientific similarities to Baculovirus Expression Vectors or soft robotics. The field has mainly been proposed because of its potential technical advantages over mammalian cells in generating cultivated meat. The name of the field was coined by Natalie Rubio at Tufts University.
References
- 1 2 "Cell Culture Basics" (PDF). Invitrogen. pp. 26–27. Retrieved 20 June 2019.
- ↑ Bruslind, Linda. "Microbial Growth." General Microbiology. Oregon State University. Web. 15 Mar. 2021. https://open.oregonstate.education/generalmicrobiology/chapter/microbial-growth/
- ↑ "Bacterial Culture Techniques." Cold Spring Harbor Laboratory. 331-32. DNA Learning Center Lab Protocols. Web. 15 Mar. 2021. http://labprotocols.dnalc.org/files/017_bacteria_culture_techniques.pdf