
A cathode is the electrode from which a conventional current leaves a polarized electrical device. This definition can be recalled by using the mnemonic CCD for Cathode Current Departs. A conventional current describes the direction in which positive charges move. Electrons have a negative electrical charge, so the movement of electrons is opposite to that of the conventional current flow. Consequently, the mnemonic cathode current departs also means that electrons flow into the device's cathode from the external circuit. For example, the end of a household battery marked with a + (plus) is the cathode.

Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference, as a measurable and quantitative phenomenon, and identifiable chemical change, with the potential difference as an outcome of a particular chemical change, or vice versa. These reactions involve electrons moving via an electronically-conducting phase between electrodes separated by an ionically conducting and electronically insulating electrolyte.

An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit. Electrodes are essential parts of batteries that can consist of a variety of materials depending on the type of battery.

An electrochemical cell is a device capable of either generating electrical energy from chemical reactions or using electrical energy to cause chemical reactions. The electrochemical cells which generate an electric current are called voltaic or galvanic cells and those that generate chemical reactions, via electrolysis for example, are called electrolytic cells. A common example of a galvanic cell is a standard 1.5 volt cell meant for consumer use. A battery consists of one or more cells, connected in parallel, series or series-and-parallel pattern.

In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from naturally occurring sources such as ores using an electrolytic cell. The voltage that is needed for electrolysis to occur is called the decomposition potential. The word "lysis" means to separate or break, so in terms, electrolysis would mean "breakdown via electricity".

A galvanic cell or voltaic cell, named after the scientists Luigi Galvani and Alessandro Volta, respectively, is an electrochemical cell in which an electric current is generated from spontaneous Oxidation-Reduction reactions. A common apparatus generally consists of two different metals, each immersed in separate beakers containing their respective metal ions in solution that are connected by a salt bridge or separated by a porous membrane.

An electrolytic cell is an electrochemical cell that utilizes an external source of electrical energy to drive a chemical reaction that would not otherwise occur. This is in contrast to a galvanic cell, which itself is a source of electrical energy and the foundation of a battery. The net reaction taking place in a galvanic cell is a spontaneous reaction, i.e, the Gibbs free energy remains negative, while the net reaction taking place in an electrolytic cell is the reverse of this spontaneous reaction, i.e, the Gibbs free energy is positive.

Paschen's law is an equation that gives the breakdown voltage, that is, the voltage necessary to start a discharge or electric arc, between two electrodes in a gas as a function of pressure and gap length. It is named after Friedrich Paschen who discovered it empirically in 1889.
The chloralkali process is an industrial process for the electrolysis of sodium chloride solutions. It is the technology used to produce chlorine and sodium hydroxide, which are commodity chemicals required by industry. 35 million tons of chlorine were prepared by this process in 1987. The chlorine and sodium hydroxide produced in this process are widely used in the chemical industry.

The alkaline fuel cell (AFC), also known as the Bacon fuel cell after its British inventor, Francis Thomas Bacon, is one of the most developed fuel cell technologies. Alkaline fuel cells consume hydrogen and pure oxygen, to produce potable water, heat, and electricity. They are among the most efficient fuel cells, having the potential to reach 70%.
A "photoelectrochemical cell" is one of two distinct classes of device. The first produces electrical energy similarly to a dye-sensitized photovoltaic cell, which meets the standard definition of a photovoltaic cell. The second is a photoelectrolytic cell, that is, a device which uses light incident on a photosensitizer, semiconductor, or aqueous metal immersed in an electrolytic solution to directly cause a chemical reaction, for example to produce hydrogen via the electrolysis of water.

Electrophoretic deposition (EPD), is a term for a broad range of industrial processes which includes electrocoating, cathodic electrodeposition, anodic electrodeposition, and electrophoretic coating, or electrophoretic painting. A characteristic feature of this process is that colloidal particles suspended in a liquid medium migrate under the influence of an electric field (electrophoresis) and are deposited onto an electrode. All colloidal particles that can be used to form stable suspensions and that can carry a charge can be used in electrophoretic deposition. This includes materials such as polymers, pigments, dyes, ceramics and metals.

Electrolysis of water, also known as electrochemical water splitting, is the process of using electricity to decompose water into oxygen and hydrogen gas by a process called electrolysis. Hydrogen gas released in this way can be used as hydrogen fuel, or remixed with the oxygen to create oxyhydrogen gas, which is used in welding and other applications.
Electrosynthesis in chemistry is the synthesis of chemical compounds in an electrochemical cell. Compared to ordinary redox reaction, electrosynthesis sometimes offers improved selectivity and yields. Electrosynthesis is actively studied as a science and also has industrial applications. Electrooxidation has potential for wastewater treatment as well.
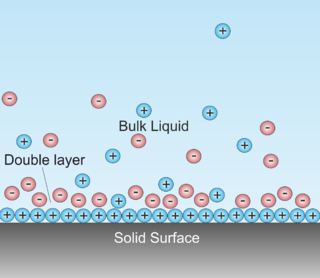
A double layer is a structure that appears on the surface of an object when it is exposed to a fluid. The object might be a solid particle, a gas bubble, a liquid droplet, or a porous body. The DL refers to two parallel layers of charge surrounding the object. The first layer, the surface charge, consists of ions adsorbed onto the object due to chemical interactions. The second layer is composed of ions attracted to the surface charge via the Coulomb force, electrically screening the first layer. This second layer is loosely associated with the object. It is made of free ions that move in the fluid under the influence of electric attraction and thermal motion rather than being firmly anchored. It is thus called the "diffuse layer".
The lithium–air battery (Li–air) is a metal–air electrochemical cell or battery chemistry that uses oxidation of lithium at the anode and reduction of oxygen at the cathode to induce a current flow.
The electrochemical regeneration of activated carbon based adsorbents involves the removal of molecules adsorbed onto the surface of the adsorbent with the use of an electric current in an electrochemical cell restoring the carbon's adsorptive capacity. Electrochemical regeneration represents an alternative to thermal regeneration commonly used in waste water treatment applications. Common adsorbents include powdered activated carbon (PAC), granular activated carbon (GAC) and activated carbon fibre.
Electrodeionization (EDI) is a water treatment technology that utilizes electricity, ion exchange membranes, and resin to deionize water and separate dissolved ions (impurities) from water. It differs from other water purification technologies in that it is done without the use of chemical treatments and is usually a polishing treatment to reverse osmosis (RO). There are also EDI units that are often referred to as continuous electrodeionization (CEDI) since the electric current regenerates the resin mass continuously. CEDI technique can achieve very high purity, with a conductivity below 0.1 μS/cm. Electrodeionization (EDI) can be differentiated into three stages, so the basics of EDI reside in the simultaneity of the following processes.
Ion transport number, also called the transference number, is the fraction of the total electrical current carried in an electrolyte by a given ionic species ,
Mixed oxidant solution is a type of disinfectant which is used for disinfecting, sterilization and eliminating pathogenic microorganisms in water and in many other applications. Using a mixed oxidant solution for water disinfection, compared to other methods, may have various benefits such as higher disinfecting power, stable residual chlorine in water, improved taste and odour, elimination of biofilm, and safety. Mixed-oxidant solution is produced by electrolysis of sodium chloride and is a mixture of disinfecting compounds. The main component of this product is chlorine and its derivatives (ClO−, HClO and Cl2 solution). It may also contain high amounts of chlorine dioxide solution, dissolved ozone, hydrogen peroxide, and oxygen, from which the name "mixed oxidant", is derived.



















