Foraminifera are single-celled organisms, members of a phylum or class of Rhizarian protists characterized by streaming granular ectoplasm for catching food and other uses; and commonly an external shell of diverse forms and materials. Tests of chitin are believed to be the most primitive type. Most foraminifera are marine, the majority of which live on or within the seafloor sediment, while a smaller number float in the water column at various depths, which belong to the suborder Globigerinina. Fewer are known from freshwater or brackish conditions, and some very few (nonaquatic) soil species have been identified through molecular analysis of small subunit ribosomal DNA.
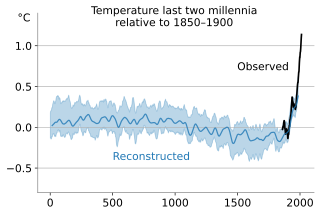
In the study of past climates ("paleoclimatology"), climate proxies are preserved physical characteristics of the past that stand in for direct meteorological measurements and enable scientists to reconstruct the climatic conditions over a longer fraction of the Earth's history. Reliable global records of climate only began in the 1880s, and proxies provide the only means for scientists to determine climatic patterns before record-keeping began.

Carbonate rocks are a class of sedimentary rocks composed primarily of carbonate minerals. The two major types are limestone, which is composed of calcite or aragonite (different crystal forms of CaCO3), and dolomite rock (also known as dolostone), which is composed of mineral dolomite (CaMg(CO3)2). They are usually classified based on texture and grain size. Importantly, carbonate rocks can exist as metamorphic and igneous rocks, too. When recrystallized carbonate rocks are metamorphosed, marble is created. Rare igneous carbonate rocks even exist as intrusive carbonatites and, even rarer, there exists volcanic carbonate lava.
A paleothermometer is a methodology that provides an estimate of the ambient temperature at the time of formation of a natural material. Most paleothermometers are based on empirically-calibrated proxy relationships, such as the tree ring or TEX86 methods. Isotope methods, such as the δ18O method or the clumped-isotope method, are able to provide, at least in theory, direct measurements of temperature.
Paleoceanography is the study of the history of the oceans in the geologic past with regard to circulation, chemistry, biology, geology and patterns of sedimentation and biological productivity. Paleoceanographic studies using environment models and different proxies enable the scientific community to assess the role of the oceanic processes in the global climate by the re-construction of past climate at various intervals. Paleoceanographic research is also intimately tied to paleoclimatology.
The environmental isotopes are a subset of isotopes, both stable and radioactive, which are the object of isotope geochemistry. They are primarily used as tracers to see how things move around within the ocean-atmosphere system, within terrestrial biomes, within the Earth's surface, and between these broad domains.

Paleolimnology is a scientific sub-discipline closely related to both limnology and paleoecology. Paleolimnological studies focus on reconstructing the past environments of inland waters using the geologic record, especially with regard to events such as climatic change, eutrophication, acidification, and internal ontogenic processes.
In geochemistry, paleoclimatology and paleoceanography δ18O or delta-O-18 is a measure of the deviation in ratio of stable isotopes oxygen-18 (18O) and oxygen-16 (16O). It is commonly used as a measure of the temperature of precipitation, as a measure of groundwater/mineral interactions, and as an indicator of processes that show isotopic fractionation, like methanogenesis. In paleosciences, 18O:16O data from corals, foraminifera and ice cores are used as a proxy for temperature.

Porites is a genus of stony coral; they are small polyp stony (SPS) corals. They are characterised by a finger-like morphology. Members of this genus have widely spaced calices, a well-developed wall reticulum and are bilaterally symmetrical. Porites, particularly Porites lutea, often form microatolls. Corals of the genus Porites also often serve as hosts for Christmas tree worms.

Sclerochronology is the study of periodic physical and chemical features in the hard tissues of animals that grow by accretion, including invertebrates and coralline red algae, and the temporal context in which they formed. It is particularly useful in the study of marine paleoclimatology. The term was coined in 1974 following pioneering work on nuclear test atolls by Knutson and Buddemeier and comes from the three Greek words skleros (hard), chronos (time) and logos (science), which together refer to the use of the hard parts of living organisms to order events in time. It is, therefore, a form of stratigraphy. Sclerochronology focuses primarily upon growth patterns reflecting annual, monthly, fortnightly, tidal, daily, and sub-daily (ultradian) increments of time.
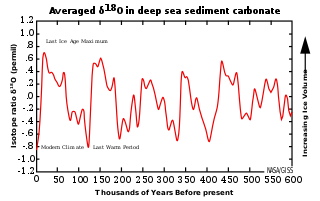
The 100,000-year problem of the Milankovitch theory of orbital forcing refers to a discrepancy between the reconstructed geologic temperature record and the reconstructed amount of incoming solar radiation, or insolation over the past 800,000 years. Due to variations in the Earth's orbit, the amount of insolation varies with periods of around 21,000, 40,000, 100,000, and 400,000 years. Variations in the amount of incident solar energy drive changes in the climate of the Earth, and are recognised as a key factor in the timing of initiation and termination of glaciations.
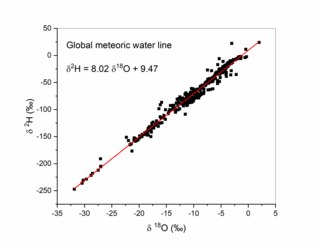
The Global Meteoric Water Line (GMWL) describes the global annual average relationship between hydrogen and oxygen isotope (oxygen-18 and deuterium) ratios in natural meteoric waters. The GMWL was first developed in 1961 by Harmon Craig, and has subsequently been widely used to track water masses in environmental geochemistry and hydrogeology.
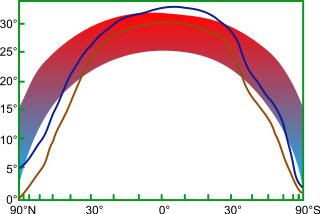
The cool tropics paradox is the apparent difference between modeled estimates of tropical temperatures during warm, ice-free periods of the Cretaceous and Eocene, and the colder temperatures which proxies suggested were present. The long-standing paradox was resolved when novel proxy derived temperatures showed significantly warmer tropics during past greenhouse climates. The low-gradient problem, i.e. the very warm polar regions with respect to present day, is still an issue for state-of-the-art climate models.
Cretaceous polar forests were temperate forests that grew at polar latitudes during the final period of the Mesozoic Era, known as the Cretaceous Period 145–66 Ma. During this period, global average temperature was about 10 °C (18 °F) higher and carbon dioxide (CO2) levels were approximately 1000 parts per million (ppm), 2.5 times the current concentration in Earth's atmosphere. The abundance of atmospheric carbon dioxide had a very significant impact on global climate and Earth's natural systems as its concentration is considered one of the main factors in the development of a pronounced greenhouse Earth during the Cretaceous, with a very low average global temperature gradient. As a consequence, high paleolatitudes in both hemispheres were much warmer than at present. This temperature gradient was partly responsible for the lack of continental ice sheets in polar regions.

The term stable isotope has a meaning similar to stable nuclide, but is preferably used when speaking of nuclides of a specific element. Hence, the plural form stable isotopes usually refers to isotopes of the same element. The relative abundance of such stable isotopes can be measured experimentally, yielding an isotope ratio that can be used as a research tool. Theoretically, such stable isotopes could include the radiogenic daughter products of radioactive decay, used in radiometric dating. However, the expression stable-isotope ratio is preferably used to refer to isotopes whose relative abundances are affected by isotope fractionation in nature. This field is termed stable isotope geochemistry.
Clumped isotopes are heavy isotopes that are bonded to other heavy isotopes. The relative abundance of clumped isotopes (and multiply-substituted isotopologues) in molecules such as methane, nitrous oxide, and carbonate is an area of active investigation. The carbonate clumped-isotope thermometer, or "13C–18O order/disorder carbonate thermometer", is a new approach for paleoclimate reconstruction, based on the temperature dependence of the clumping of 13C and 18O into bonds within the carbonate mineral lattice. This approach has the advantage that the 18O ratio in water is not necessary (different from the δ18O approach), but for precise paleotemperature estimation, it also needs very large and uncontaminated samples, long analytical runs, and extensive replication. Commonly used sample sources for paleoclimatological work include corals, otoliths, gastropods, tufa, bivalves, and foraminifera. Results are usually expressed as Δ47 (said as "cap 47"), which is the deviation of the ratio of isotopologues of CO2 with a molecular weight of 47 to those with a weight of 44 from the ratio expected if they were randomly distributed.

Marine biogenic calcification is the production of calcium carbonate by organisms in the global ocean.
Carbonate-associated sulfates (CAS) are sulfate species found in association with carbonate minerals, either as inclusions, adsorbed phases, or in distorted sites within the carbonate mineral lattice. It is derived primarily from dissolved sulfate in the solution from which the carbonate precipitates. In the ocean, the source of this sulfate is a combination of riverine and atmospheric inputs, as well as the products of marine hydrothermal reactions and biomass remineralisation. CAS is a common component of most carbonate rocks, having concentrations in the parts per thousand within biogenic carbonates and parts per million within abiogenic carbonates. Through its abundance and sulfur isotope composition, it provides a valuable record of the global sulfur cycle across time and space.

Tessa Michelle Hill is an American marine geochemist and oceanographer. She is a professor at the University of California, Davis, and a resident professor at its Bodega Marine Laboratory. She is a Fellow of the California Academy of Sciences, and in 2016 was named a Leshner Public Engagement Fellow of the American Association for the Advancement of Science. In that year she also received the US Presidential Early Career Award for Scientists and Engineers (PECASE).
Global paleoclimate indicators are the proxies sensitive to global paleoclimatic environment changes. They are mostly derived from marine sediments. Paleoclimate indicators derived from terrestrial sediments, on the other hand, are commonly influenced by local tectonic movements and paleogeographic variations. Factors governing the Earth's climate system include plate tectonics, which controls the configuration of continents, the interplay between the atmosphere and the ocean, and the Earth's orbital characteristics. Global paleoclimate indicators are established based on the information extracted from the analyses of geologic materials, including biological, geochemical and mineralogical data preserved in marine sediments. Indicators are generally grouped into three categories; paleontological, geochemical and lithological.










