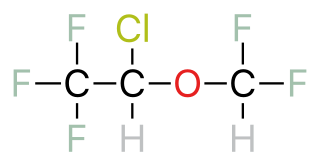General anaesthetics are often defined as compounds that induce a loss of consciousness in humans or loss of righting reflex in animals. Clinical definitions are also extended to include an induced coma that causes lack of awareness to painful stimuli, sufficient to facilitate surgical applications in clinical and veterinary practice. General anaesthetics do not act as analgesics and should also not be confused with sedatives. General anaesthetics are a structurally diverse group of compounds whose mechanisms encompasses multiple biological targets involved in the control of neuronal pathways. The precise workings are the subject of some debate and ongoing research.

Imperial Chemical Industries (ICI) was a British chemical company. It was, for much of its history, the largest manufacturer in Britain. It was formed by the merger of four leading British chemical companies in 1926. Its headquarters were at Millbank in London. ICI was a constituent of the FT 30 and later the FTSE 100 indices.

Halothane, sold under the brand name Fluothane among others, is a general anaesthetic. It can be used to induce or maintain anaesthesia. One of its benefits is that it does not increase the production of saliva, which can be particularly useful in those who are difficult to intubate. It is given by inhalation.

The chemical compound trichloroethylene (TCE) is a halocarbon with the formula C2HCl3, commonly used as an industrial solvent. It is a clear, colourless non-flammable liquid with a chloroform-like sweet smell. It should not be confused with the similar 1,1,1-trichloroethane, which is commonly known as chlorothene.

Isoflurane, sold under the brand name Forane among others, is a general anesthetic. It can be used to start or maintain anesthesia; however, other medications are often used to start anesthesia, due to airway irritation with isoflurane. Isoflurane is given via inhalation.

Sevoflurane, sold under the brand name Sevorane, among others, is a sweet-smelling, nonflammable, highly fluorinated methyl isopropyl ether used as an inhalational anaesthetic for induction and maintenance of general anesthesia. After desflurane, it is the volatile anesthetic with the fastest onset. While its offset may be faster than agents other than desflurane in a few circumstances, its offset is more often similar to that of the much older agent isoflurane. While sevoflurane is only half as soluble as isoflurane in blood, the tissue blood partition coefficients of isoflurane and sevoflurane are quite similar. For example, in the muscle group: isoflurane 2.62 vs. sevoflurane 2.57. In the fat group: isoflurane 52 vs. sevoflurane 50. As a result, the longer the case, the more similar will be the emergence times for sevoflurane and isoflurane.

An anesthetic or anaesthetic is a drug used to induce anesthesia — in other words, to result in a temporary loss of sensation or awareness. They may be divided into two broad classes: general anesthetics, which result in a reversible loss of consciousness, and local anesthetics, which cause a reversible loss of sensation for a limited region of the body without necessarily affecting consciousness.
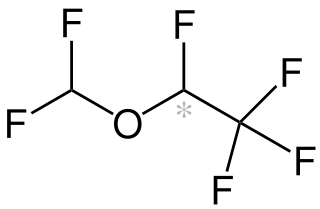
Desflurane (1,2,2,2-tetrafluoroethyl difluoromethyl ether) is a highly fluorinated methyl ethyl ether used for maintenance of general anesthesia. Like halothane, enflurane, and isoflurane, it is a racemic mixture of (R) and (S) optical isomers (enantiomers). Together with sevoflurane, it is gradually replacing isoflurane for human use, except in economically undeveloped areas, where its high cost precludes its use. It has the most rapid onset and offset of the volatile anesthetic drugs used for general anesthesia due to its low solubility in blood.

An inhalational anesthetic is a chemical compound possessing general anesthetic properties that can be delivered via inhalation. They are administered through a face mask, laryngeal mask airway or tracheal tube connected to an anesthetic vaporiser and an anesthetic delivery system. Agents of significant contemporary clinical interest include volatile anesthetic agents such as isoflurane, sevoflurane and desflurane, as well as certain anesthetic gases such as nitrous oxide and xenon.
A halogenated ether is a subcategory of a larger group of chemicals known as ethers. An ether is an organic chemical that contains an ether group—an oxygen atom connected to two (substituted) alkyl groups. A good example of an ether is the solvent diethyl ether.
Minimum alveolar concentration or MAC is the concentration, often expressed as a percentage by volume, of a vapour in the alveoli of the lungs that is needed to prevent movement in 50% of subjects in response to surgical (pain) stimulus. MAC is used to compare the strengths, or potency, of anaesthetic vapours. The concept of MAC was first introduced in 1965.
Charles Walter Suckling was a British chemist who first synthesised halothane, a volatile inhalational anaesthetic in 1951, while working at the Imperial Chemical Industries (ICI) Central Laboratory in Widnes.

Oldershaw Academy is a secondary school located in the Liscard area of Wallasey, England, and is a specialist Business and Enterprise College.
The chemical agent used in the Moscow theatre hostage crisis of 23 October 2002 has never been definitively revealed by the Russian authorities, though many possible identities have been speculated. An undisclosed incapacitating agent was used by the Russian authorities in order to subdue the Chechen terrorists who had taken control of a crowded theater.
A scavenger system is a medical device used in hospitals. It is used to gather gas or aerosolized medication after it is exhaled from the patient or left the area of the patient. Often used to collect anesthesia, it can also be used to collect any type of gas or aerosolized medicine that is intended only for the patient and should not be breathed in by any other medical personnel.

An anesthetic vaporizer or anaesthetic vaporiser is a device generally attached to an anesthetic machine which delivers a given concentration of a volatile anesthetic agent. It works by controlling the vaporization of anesthetic agents from liquid, and then accurately controlling the concentration in which these are added to the fresh gas flow. The design of these devices takes account of varying: ambient temperature, fresh gas flow, and agent vapor pressure.

The Schimmelbusch mask is an open breathing system for delivering an anesthetic. The device was invented by Curt Schimmelbusch in 1889, and was used until the 1950s. The device consists of a wire frame which is covered with several beds of gauze and applied to the patient's face over the mouth and nose. Then high-volatility anesthetic is dripped on it, allowing the patient to inhale a mix of the evaporated anesthetic and air. The device is designed to prevent the anesthetic from coming in contact with the patient's skin, where it can cause irritation.
Blood–gas partition coefficient, also known as Ostwald coefficient for blood–gas, is a term used in pharmacology to describe the solubility of inhaled general anesthetics in blood. According to Henry's law, the ratio of the concentration in blood to the concentration in gas that is in contact with that blood, when the partial pressure in both compartments is equal, is nearly constant at sufficiently low concentrations. The partition coefficient is defined as this ratio and, therefore, has no units. The concentration of the anesthetic in blood includes the portion that is undissolved in plasma and the portion that is dissolved. The more soluble the inhaled anesthetic is in blood compared to in air, the more it binds to plasma proteins in the blood and the higher the blood–gas partition coefficient.
Sir James Gordon Robson was a Scottish anaesthetist

The Winnington Laboratory was a former chemical laboratory at Winnington, near Northwich, in Cheshire, England.