Related Research Articles

Analytical chemistry studies and uses instruments and methods to separate, identify, and quantify matter. In practice, separation, identification or quantification may constitute the entire analysis or be combined with another method. Separation isolates analytes. Qualitative analysis identifies analytes, while quantitative analysis determines the numerical amount or concentration.

Titration is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte. A reagent, termed the titrant or titrator, is prepared as a standard solution of known concentration and volume. The titrant reacts with a solution of analyte to determine the analyte's concentration. The volume of titrant that reacted with the analyte is termed the titration volume.

Ultrasound is sound with frequencies greater than 20 kilohertz. This frequency is the approximate upper audible limit of human hearing in healthy young adults. The physical principles of acoustic waves apply to any frequency range, including ultrasound. Ultrasonic devices operate with frequencies from 20 kHz up to several gigahertz.
Instrumentation is a collective term for measuring instruments, used for indicating, measuring, and recording physical quantities. It is also a field of study about the art and science about making measurement instruments, involving the related areas of metrology, automation, and control theory. The term has its origins in the art and science of scientific instrument-making.

Ultraviolet (UV) spectroscopy or ultraviolet–visible (UV–VIS) spectrophotometry refers to absorption spectroscopy or reflectance spectroscopy in part of the ultraviolet and the full, adjacent visible regions of the electromagnetic spectrum. Being relatively inexpensive and easily implemented, this methodology is widely used in diverse applied and fundamental applications. The only requirement is that the sample absorb in the UV-Vis region, i.e. be a chromophore. Absorption spectroscopy is complementary to fluorescence spectroscopy. Parameters of interest, besides the wavelength of measurement, are absorbance (A) or transmittance (%T) or reflectance (%R), and its change with time.

Differential scanning calorimetry (DSC) is a thermoanalytical technique in which the difference in the amount of heat required to increase the temperature of a sample and reference is measured as a function of temperature. Both the sample and reference are maintained at nearly the same temperature throughout the experiment. Generally, the temperature program for a DSC analysis is designed such that the sample holder temperature increases linearly as a function of time. The reference sample should have a well-defined heat capacity over the range of temperatures to be scanned. Additionally, the reference sample must be stable, of high purity, and must not experience much change across the temperature scan. Typically, reference standards have been metals such as indium, tin, bismuth, and lead, but other standards such as polyethylene and fatty acids have been proposed to study polymers and organic compounds, respectively.
Chemometrics is the science of extracting information from chemical systems by data-driven means. Chemometrics is inherently interdisciplinary, using methods frequently employed in core data-analytic disciplines such as multivariate statistics, applied mathematics, and computer science, in order to address problems in chemistry, biochemistry, medicine, biology and chemical engineering. In this way, it mirrors other interdisciplinary fields, such as psychometrics and econometrics.

Absorption spectroscopy is spectroscopy that involves techniques that measure the absorption of electromagnetic radiation, as a function of frequency or wavelength, due to its interaction with a sample. The sample absorbs energy, i.e., photons, from the radiating field. The intensity of the absorption varies as a function of frequency, and this variation is the absorption spectrum. Absorption spectroscopy is performed across the electromagnetic spectrum.
An assay is an investigative (analytic) procedure in laboratory medicine, mining, pharmacology, environmental biology and molecular biology for qualitatively assessing or quantitatively measuring the presence, amount, or functional activity of a target entity. The measured entity is often called the analyte, the measurand, or the target of the assay. The analyte can be a drug, biochemical substance, chemical element or compound, or cell in an organism or organic sample. An assay usually aims to measure an analyte's intensive property and express it in the relevant measurement unit.

In analytical chemistry, a calibration curve, also known as a standard curve, is a general method for determining the concentration of a substance in an unknown sample by comparing the unknown to a set of standard samples of known concentration. A calibration curve is one approach to the problem of instrument calibration; other standard approaches may mix the standard into the unknown, giving an internal standard. The calibration curve is a plot of how the instrumental response, the so-called analytical signal, changes with the concentration of the analyte.
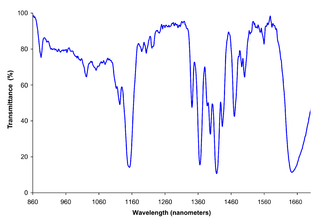
Near-infrared spectroscopy (NIRS) is a spectroscopic method that uses the near-infrared region of the electromagnetic spectrum. Typical applications include medical and physiological diagnostics and research including blood sugar, pulse oximetry, functional neuroimaging, sports medicine, elite sports training, ergonomics, rehabilitation, neonatal research, brain computer interface, urology, and neurology. There are also applications in other areas as well such as pharmaceutical, food and agrochemical quality control, atmospheric chemistry, combustion research and knowledge.

Ion chromatography is a form of chromatography that separates ions and ionizable polar molecules based on their affinity to the ion exchanger. It works on almost any kind of charged molecule—including small inorganic anions, large proteins, small nucleotides, and amino acids. However, ion chromatography must be done in conditions that are one pH unit away from the isoelectric point of a protein.
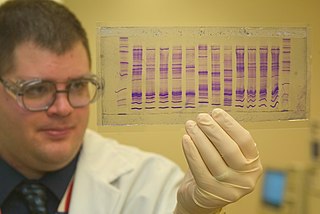
Forensic chemistry is the application of chemistry and its subfield, forensic toxicology, in a legal setting. A forensic chemist can assist in the identification of unknown materials found at a crime scene. Specialists in this field have a wide array of methods and instruments to help identify unknown substances. These include high-performance liquid chromatography, gas chromatography-mass spectrometry, atomic absorption spectroscopy, Fourier transform infrared spectroscopy, and thin layer chromatography. The range of different methods is important due to the destructive nature of some instruments and the number of possible unknown substances that can be found at a scene. Forensic chemists prefer using nondestructive methods first, to preserve evidence and to determine which destructive methods will produce the best results.
The limit of detection is the lowest signal, or the lowest corresponding quantity to be determined from the signal, that can be observed with a sufficient degree of confidence or statistical significance. However, the exact threshold used to decide when a signal significantly emerges above the continuously fluctuating background noise remains arbitrary and is a matter of policy and often of debate among scientists, statisticians and regulators depending on the stakes in different fields.
Polarography is a type of voltammetry where the working electrode is a dropping mercury electrode (DME) or a static mercury drop electrode (SMDE), which are useful for their wide cathodic ranges and renewable surfaces. It was invented in 1922 by Czechoslovak chemist Jaroslav Heyrovský, for which he won the Nobel prize in 1959. The main advantages of mercury as electrode material are as follows: 1) a large voltage window: ca. from +0.2 V to -1.8 V vs reversible hydrogen electrode (RHE). Hg electrode is particularly well-suited for studying electroreduction reactions. 2) very reproducible electrode surface, since mercury is liquid. 3) very easy cleaning of the electrode surface by making a new drop of mercury from a large Hg pool connected by a glass capillary.
Laboratory quality control is designed to detect, reduce, and correct deficiencies in a laboratory's internal analytical process prior to the release of patient results, in order to improve the quality of the results reported by the laboratory. Quality control (QC) is a measure of precision, or how well the measurement system reproduces the same result over time and under varying operating conditions. Laboratory quality control material is usually run at the beginning of each shift, after an instrument is serviced, when reagent lots are changed, after equipment calibration, and whenever patient results seem inappropriate. Quality control material should approximate the same matrix as patient specimens, taking into account properties such as viscosity, turbidity, composition, and color. IIt should be stable for long periods of time, and available in large enough quantities for a single batch to last at least one year. Liquid controls are more convenient than lyophilized (freeze-dried) controls because they do not have to be reconstituted, minimizing pipetting error. Dried Tube Specimen (DTS) is slightly cumbersome as a QC material but it is very low-cost, stable over long periods and efficient, especially useful for resource-restricted settings in under-developed and developing countries. DTS can be manufactured in-house by a laboratory or Blood Bank for its use.
Acoustic resonance spectroscopy (ARS) is a method of spectroscopy in the acoustic region, primarily the sonic and ultrasonic regions. ARS is typically much more rapid than HPLC and NIR. It is non destructive and requires no sample preparation as the sampling waveguide can simply be pushed into a sample powder/liquid or in contact with a solid sample.
Environmental monitoring describes the processes and activities that need to take place to characterize and monitor the quality of the environment. Environmental monitoring is used in the preparation of environmental impact assessments, as well as in many circumstances in which human activities carry a risk of harmful effects on the natural environment. All monitoring strategies and programs have reasons and justifications which are often designed to establish the current status of an environment or to establish trends in environmental parameters. In all cases, the results of monitoring will be reviewed, analyzed statistically, and published. The design of a monitoring program must therefore have regard to the final use of the data before monitoring starts.
Radioanalytical chemistry focuses on the analysis of sample for their radionuclide content. Various methods are employed to purify and identify the radioelement of interest through chemical methods and sample measurement techniques.
Psychoacoustics is the branch of psychophysics involving the scientific study of sound perception and audiology—how the human auditory system perceives various sounds. More specifically, it is the branch of science studying the psychological responses associated with sound. Psychoacoustics is an interdisciplinary field including psychology, acoustics, electronic engineering, physics, biology, physiology, and computer science.
References
- ↑ Wenclawiak, Bernd W.; Koch, Michael; Hadjicostas, Evsevios (2010-08-05). Quality Assurance in Analytical Chemistry: Training and Teaching. Springer Science & Business Media. p. 229. ISBN 978-3-642-13609-2.
- ↑ Reichenbächer, Manfred; Einax, Jürgen W. (2011-02-16). Challenges in Analytical Quality Assurance. Springer Science & Business Media. p. 108. ISBN 978-3-642-16595-5.