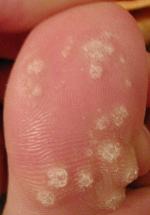
Warts are typically small, rough, hard growths that are similar in color to the rest of the skin. They typically do not result in other symptoms, except when on the bottom of the feet, where they may be painful. While they usually occur on the hands and feet, they can also affect other locations. One or many warts may appear. They are not cancerous.

Cervical cancer is a cancer arising from the cervix. It is due to the abnormal growth of cells that have the ability to invade or spread to other parts of the body. Early on, typically no symptoms are seen. Later symptoms may include abnormal vaginal bleeding, pelvic pain or pain during sexual intercourse. While bleeding after sex may not be serious, it may also indicate the presence of cervical cancer.
Human papillomavirus infection is caused by a DNA virus from the Papillomaviridae family. Many HPV infections cause no symptoms and 90% resolve spontaneously within two years. However, in some cases, an HPV infection persists and results in either warts or precancerous lesions. These lesions, depending on the site affected, increase the risk of cancer of the cervix, vulva, vagina, penis, anus, mouth, tonsils, or throat. Nearly all cervical cancer is due to HPV; two strains, HPV16 and HPV18, account for 70% of cases. HPV16 is responsible for almost 90% of HPV-positive oropharyngeal cancers. Between 60% and 90% of the other cancers listed above are also linked to HPV. HPV6 and HPV11 are common causes of genital warts and laryngeal papillomatosis.

Genital warts are a sexually transmitted infection caused by certain types of human papillomavirus (HPV). They are generally pink in color and project out from the surface of the skin. Usually they cause few symptoms, but can occasionally be painful. Typically they appear one to eight months following exposure. Warts are the most easily recognized symptom of genital HPV infection.

Hepatitis E is inflammation of the liver caused by infection with the hepatitis E virus (HEV); it is a type of viral hepatitis. Hepatitis E has mainly a fecal-oral transmission route that is similar to hepatitis A, although the viruses are unrelated. In retrospect, the earliest known epidemic of hepatitis E occurred in 1955 in New Delhi, but the virus was not isolated until 1983 by Russian scientists investigating an outbreak in Afghanistan. HEV is a positive-sense, single-stranded, nonenveloped, RNA icosahedral virus and one of five known human hepatitis viruses: A, B, C, D, and E.

Papillomaviridae is a family of non-enveloped DNA viruses whose members are known as papillomaviruses. Several hundred species of papillomaviruses, traditionally referred to as "types", have been identified infecting all carefully inspected mammals, but also other vertebrates such as birds, snakes, turtles and fish. Infection by most papillomavirus types, depending on the type, is either asymptomatic or causes small benign tumors, known as papillomas or warts. Papillomas caused by some types, however, such as human papillomaviruses 16 and 18, carry a risk of becoming cancerous.

Anal cancer is a cancer which arises from the anus, the distal opening of the gastrointestinal tract. Symptoms may include bleeding from the anus or a lump near the anus. Other symptoms may include pain, itchiness, or discharge from the anus. A change in bowel movements may also occur.
An oncovirus or oncogenic virus is a virus that can cause cancer. This term originated from studies of acutely transforming retroviruses in the 1950–60s, when the term "oncornaviruses" was used to denote their RNA virus origin. With the letters "RNA" removed, it now refers to any virus with a DNA or RNA genome causing cancer and is synonymous with "tumor virus" or "cancer virus". The vast majority of human and animal viruses do not cause cancer, probably because of longstanding co-evolution between the virus and its host. Oncoviruses have been important not only in epidemiology, but also in investigations of cell cycle control mechanisms such as the retinoblastoma protein.
Virus-like particles (VLPs) are molecules that closely resemble viruses, but are non-infectious because they contain no viral genetic material. They can be naturally occurring or synthesized through the individual expression of viral structural proteins, which can then self assemble into the virus-like structure. Combinations of structural capsid proteins from different viruses can be used to create recombinant VLPs. VLPs derived from the Hepatitis B virus (HBV) and composed of the small HBV derived surface antigen (HBsAg) were described in 1968 from patient sera. VLPs have been produced from components of a wide variety of virus families including Parvoviridae, Retroviridae, Flaviviridae, Paramyxoviridae and bacteriophages. VLPs can be produced in multiple cell culture systems including bacteria, mammalian cell lines, insect cell lines, yeast and plant cells.

Cervical intraepithelial neoplasia (CIN), also known as cervical dysplasia, is the abnormal growth of cells on the surface of the cervix that could potentially lead to cervical cancer. More specifically, CIN refers to the potentially precancerous transformation of cells of the cervix.

Human papillomavirus (HPV) vaccines are vaccines that prevent infection by certain types of human papillomavirus (HPV). Available HPV vaccines protect against either two, four, or nine types of HPV. All HPV vaccines protect against at least HPV types 16 and 18, which cause the greatest risk of cervical cancer. It is estimated that HPV vaccines may prevent 70% of cervical cancer, 80% of anal cancer, 60% of vaginal cancer, 40% of vulvar cancer, and show more than 90% efficacy in preventing HPV-positive oropharyngeal cancers. They additionally prevent some genital warts, with the quadrivalent and nonavalent vaccines that protect against HPV types HPV-6 and HPV-11 providing greater protection.

Gardasil, technically known as recombinant human papillomavirus vaccine [types 6, 11, 16, 18], is a vaccine for use in the prevention of certain strains of human papillomavirus (HPV), developed by Merck & Co. High-risk human papilloma virus (hr-HPV) genital infection is the most common sexually transmitted infection among women. The HPV strains that Gardasil protects against are sexually transmitted, specifically HPV types 6, 11, 16 and 18. HPV types 16 and 18 cause an estimated 70% of cervical cancers, and are responsible for most HPV-induced anal, vulvar, vaginal, and penile cancer cases. HPV types 6 and 11 cause an estimated 90% of genital warts cases. HPV type 16 is responsible for almost 90% of HPV-positive oropharyngeal cancers, and the prevalence is higher in males than females. Though Gardasil does not treat existing infection, vaccination is still recommended for HPV-positive individuals, as it may protect against one or more different strains of the disease.

Bovine papillomaviruses (BPV) are a paraphyletic group of DNA viruses of the subfamily Firstpapillomavirinae of Papillomaviridae that are common in cattle. All BPVs have a circular double-stranded DNA genome. Infection causes warts of the skin and alimentary tract, and more rarely cancers of the alimentary tract and urinary bladder. They are also thought to cause the skin tumour equine sarcoid in horses and donkeys.
Cervarix is a vaccine against certain types of cancer-causing human papillomavirus (HPV).
Margaret Anne Stanley, OBE FMedSci is a British virologist and epithelial biologist. She attended the Universities of London, Bristol, and Adelaide. As of 2018, she is an Emeritus Professor of Epithelial Biology in the Department of Pathology at University of Cambridge and a Fellow of the Academy of Medical Sciences. She is also an Honorary Fellow of the UK Royal College of Obstetricians and Gynaecologists and an Honorary Fellow of Christ's College, Cambridge. Stanley is a research scientist in the field of virology with particular focus on the human papillomavirus (HPV). Her research work has led to new scientific findings on HPV. Additionally, she uses her expertise on HPV to serve on multiple different advisory committees and journal editorial boards.

Nventa Biopharmaceuticals Corporation was a Canadian-incorporated biopharmaceutical company headquartered in San Diego, California developing therapeutics for the treatment of viral infections and cancer, focusing on diseases caused by human papillomavirus (HPV). Nventa is currently the only company applying heat shock protein (Hsp) technology to target the over 20 million Americans already infected with HPV. Previously headquartered in Victoria, British Columbia, Canada, the company’s common stock traded on the Toronto Stock Exchange under the symbol: NVN.
HspE7 is an investigational therapeutic vaccine candidate being developed by Nventa Biopharmaceuticals for the treatment of precancerous and cancerous lesions caused by the human papillomavirus (HPV). HspE7 uses recombinant DNA technology to covalently fuse a heat shock protein (Hsp) to a target antigen, thereby stimulating cellular immune system responses to specific diseases. HspE7 is a patented construct consisting of the HPV Type 16 E7 protein and heat shock protein 65 (Hsp65) and is currently the only candidate using Hsp technology to target the over 20 million Americans already infected with HPV.
A hepatitis C vaccine, a vaccine capable of protecting against the hepatitis C virus (HCV), is not yet available. Although vaccines exist for hepatitis A and hepatitis B, development of an HCV vaccine has presented challenges. No vaccine is currently available, but several vaccines are currently under development.

Human papillomavirus-positive oropharyngeal cancer, is a cancer of the throat caused by the human papillomavirus type 16 virus (HPV16). In the past, cancer of the oropharynx (throat) was associated with the use of alcohol or tobacco or both, but the majority of cases are now associated with the HPV virus, acquired by having oral contact with the genitals of a person who has a genital HPV infection. Risk factors include having a large number of sexual partners, a history of oral-genital sex or anal–oral sex, having a female partner with a history of either an abnormal Pap smear or cervical dysplasia, having chronic periodontitis, and, among men, younger age at first intercourse and a history of genital warts. HPV-positive OPC is considered a separate disease from HPV-negative oropharyngeal cancer.
Anna-Lise WilliamsonMASSAf is a Professor of Virology at the University of Cape Town. Williamson obtained her PhD from the University of the Witwatersrand in 1985. Her area of expertise is human papillomavirus, but is also known on an international level for her work in developing vaccines for HIV. These vaccines have been introduce in phase 1 of clinical trial. Williamson has published more than 120 papers.












