
Low-density lipoprotein (LDL) is one of the five major groups of lipoprotein which transport all fat molecules around the body in the extracellular water. These groups, from least dense, compared to surrounding water to most dense, are chylomicrons, very low-density lipoprotein (VLDL), intermediate-density lipoprotein (IDL), low-density lipoprotein and high-density lipoprotein (HDL). LDL delivers fat molecules to the cells and can drive the progression of atherosclerosis if they become oxidized within the walls of arteries.

A lipoprotein is a biochemical assembly whose primary purpose is to transport hydrophobic lipid molecules in water, as in blood or extracellular fluid. They have a single-layer phospholipid and cholesterol outer shell, with the hydrophilic portions oriented outward toward the surrounding water and lipophilic portions of each molecule oriented inwards toward the lipids molecules within the particles. Apolipoproteins are embedded in the membrane, both stabilising the complex and giving it functional identity determining its fate. Thus the complex serves to emulsify the fats. Many enzymes, transporters, structural proteins, antigens, adhesins, and toxins are lipoproteins. Examples include the plasma lipoprotein particles classified as HDL, LDL, IDL, VLDL and ULDL lipoproteins, according to density / size, compared with the surrounding plasma water. These complex protein capsules enable fats to be carried in all extracellular water, including the blood stream, subgroups of which are primary drivers / modulators of atherosclerosis, the transmembrane proteins of mitochondrion, chloroplast, and bacterial lipoproteins. Proteolipids are a different kind of protein-lipid combination that are insoluble in water. Proteolipids are abundant in brain tissue, and are also present in many other animal and plant tissues.
Intermediate-density lipoproteins (IDLs) belong to the lipoprotein particle family and are formed from the degradation of very low-density lipoproteins as well as high-density lipoproteins. IDL is one of the five major groups of lipoproteins that enable fats and cholesterol to move within the water-based solution of the bloodstream. Each native IDL particle consists of protein that encircles various lipids, enabling, as a water-soluble particle, these lipids to travel in the aqueous blood environment as part of the fat transport system within the body. Their size is, in general, 25 to 35 nm in diameter, and they contain primarily a range of triacylglycerols and cholesterol esters. They are cleared from the plasma into the liver by receptor-mediated endocytosis, or further degraded by hepatic lipase to form LDL particles.

Apolipoproteins are proteins that bind lipids to form lipoproteins. They transport lipids in blood, cerebrospinal fluid and lymph.

Michael Stuart Brown ForMemRS is an American geneticist and Nobel laureate. He was awarded the Nobel Prize in Physiology or Medicine with Joseph L. Goldstein in 1985 for describing the regulation of cholesterol metabolism.

The Low-Density Lipoprotein (LDL) Receptor (LDL-R) is a mosaic protein of 839 amino acids that mediates the endocytosis of cholesterol-rich LDL. It is a cell-surface receptor that recognizes the apoprotein B100, which is embedded in the outer phospholipid layer of LDL particles. The receptor also recognizes the apoE protein found in chylomicron remnants and VLDL remnants (IDL). In humans, the LDL receptor protein is encoded by the LDLR gene on chromosome 19. It belongs to the Low density lipoprotein receptor gene family. It is most significantly expressed in bronchial epithelial cells and adrenal gland and cortex tissue.

In structural biology, a beta-propeller is a type of all-β protein architecture characterized by 4 to 8 highly symmetrical blade-shaped beta sheets arranged toroidally around a central axis. Together the beta-sheets form a funnel-like active site.

The low-density lipoprotein receptor gene family codes for a class of structurally related cell surface receptors that fulfill diverse biological functions in different organs, tissues, and cell types. The role that is most commonly associated with this evolutionarily ancient family is cholesterol homeostasis. In humans, excess cholesterol in the blood is captured by low-density lipoprotein (LDL) and removed by the liver via endocytosis of the LDL receptor. Recent evidence indicates that the members of the LDL receptor gene family are active in the cell signalling pathways between specialized cells in many, if not all, multicellular organisms.

The very-low-density-lipoprotein receptor (VLDLR) is a transmembrane lipoprotein receptor of the low-density-lipoprotein (LDL) receptor family. VLDLR shows considerable homology with the members of this lineage. Discovered in 1992 by T. Yamamoto, VLDLR is widely distributed throughout the tissues of the body, including the heart, skeletal muscle, adipose tissue, and the brain, but is absent from the liver. This receptor has an important role in cholesterol uptake, metabolism of apoprotein-E-containing triacylglycerol-rich lipoproteins, and neuronal migration in the developing brain. In humans, VLDLR is encoded by the VLDLR gene. Mutations of this gene may lead to a variety of symptoms and diseases, which include type I lissencephaly, cerebellar hypoplasia, and atherosclerosis.
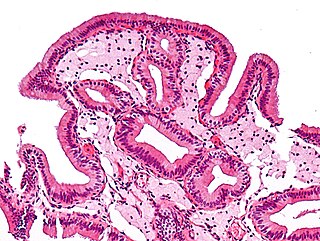
Foam cells are the fat-laden M2 macrophages that serve as the hallmark of early stage atherosclerotic lesion formation. As these plaques mature, they become more unstable and inflamed; blood clots triggered when an unstable plaque ruptures are the main driver of heart attack and stroke.

A leucine-rich repeat (LRR) is a protein structural motif that forms an α/β horseshoe fold. It is composed of repeating 20–30 amino acid stretches that are unusually rich in the hydrophobic amino acid leucine. These tandem repeats commonly fold together to form a solenoid protein domain, termed leucine-rich repeat domain. Typically, each repeat unit has beta strand-turn-alpha helix structure, and the assembled domain, composed of many such repeats, has a horseshoe shape with an interior parallel beta sheet and an exterior array of helices. One face of the beta sheet and one side of the helix array are exposed to solvent and are therefore dominated by hydrophilic residues. The region between the helices and sheets is the protein's hydrophobic core and is tightly sterically packed with leucine residues.

Familial hypercholesterolemia (FH) is a genetic disorder characterized by high cholesterol levels, specifically very high levels of low-density lipoprotein, in the blood and early cardiovascular disease. Since the underlying body biochemistry is slightly different in individuals with FH, their high cholesterol levels are less responsive to the kinds of cholesterol control methods which are usually more effective in people without FH. Nevertheless, treatment is usually effective.

Low density lipoprotein receptor-related protein-associated protein 1 also known as LRPAP1 or RAP is a chaperone protein which in humans is encoded by the LRPAP1 gene.
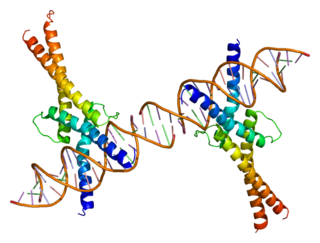
Sterol regulatory element-binding transcription factor 1 (SREBF1) also known as sterol regulatory element-binding protein 1 (SREBP-1) is a protein that in humans is encoded by the SREBF1 gene.

Oxidized low-density lipoprotein receptor 1 also known as lectin-type oxidized LDL receptor 1 (LOX-1) is a protein that in humans is encoded by the OLR1 gene.

Low density lipoprotein receptor-related protein 1 (LRP1), also known as alpha-2-macroglobulin receptor (A2MR), apolipoprotein E receptor (APOER) or cluster of differentiation 91 (CD91), is a protein forming a receptor found in the plasma membrane of cells involved in receptor-mediated endocytosis. In humans, the LRP1 protein is encoded by the LRP1 gene. LRP1 is also a key signalling protein and, thus, involved in various biological processes, such as lipoprotein metabolism and cell motility, and diseases, such as neurodegenerative diseases, atherosclerosis, and cancer.

Proprotein convertase subtilisin/kexin type 9 (PCSK9) is an enzyme encoded by the PCSK9 gene in humans on chromosome 1. It is the 9th member of the proprotein convertase family of proteins that activate other proteins. Similar genes (orthologs) are found across many species. As with many proteins, PCSK9 is inactive when first synthesized, because a section of peptide chains blocks their activity; proprotein convertases remove that section to activate the enzyme. The PCSK9 gene also contains one of 27 loci associated with increased risk of coronary artery disease.

The EGF-like domain is an evolutionary conserved protein domain, which derives its name from the epidermal growth factor where it was first described. It comprises about 30 to 40 amino-acid residues and has been found in a large number of mostly animal proteins. Most occurrences of the EGF-like domain are found in the extracellular domain of membrane-bound proteins or in proteins known to be secreted. An exception to this is the prostaglandin-endoperoxide synthase. The EGF-like domain includes 6 cysteine residues which in the epidermal growth factor have been shown to form 3 disulfide bonds. The structures of 4-disulfide EGF-domains have been solved from the laminin and integrin proteins. The main structure of EGF-like domains is a two-stranded β-sheet followed by a loop to a short C-terminal, two-stranded β-sheet. These two β-sheets are usually denoted as the major (N-terminal) and minor (C-terminal) sheets. EGF-like domains frequently occur in numerous tandem copies in proteins: these repeats typically fold together to form a single, linear solenoid domain block as a functional unit.

Low-density lipoprotein receptor-related protein 6 is a protein that in humans is encoded by the LRP6 gene. LRP6 is a key component of the LRP5/LRP6/Frizzled co-receptor group that is involved in canonical Wnt pathway.





















