Related Research Articles

Yeasts are eukaryotic, single-celled microorganisms classified as members of the fungus kingdom. The first yeast originated hundreds of millions of years ago, and at least 1,500 species are currently recognized. They are estimated to constitute 1% of all described fungal species.

Mannose is a sugar monomer of the aldohexose series of carbohydrates. It is a C-2 epimer of glucose. Mannose is important in human metabolism, especially in the glycosylation of certain proteins. Several congenital disorders of glycosylation are associated with mutations in enzymes involved in mannose metabolism.

Saccharomyces cerevisiae is a species of yeast. The species has been instrumental in winemaking, baking, and brewing since ancient times. It is believed to have been originally isolated from the skin of grapes. It is one of the most intensively studied eukaryotic model organisms in molecular and cell biology, much like Escherichia coli as the model bacterium. It is the microorganism which causes many common types of fermentation. S. cerevisiae cells are round to ovoid, 5–10 μm in diameter. It reproduces by budding.

Schizosaccharomyces pombe, also called "fission yeast", is a species of yeast used in traditional brewing and as a model organism in molecular and cell biology. It is a unicellular eukaryote, whose cells are rod-shaped. Cells typically measure 3 to 4 micrometres in diameter and 7 to 14 micrometres in length. Its genome, which is approximately 14.1 million base pairs, is estimated to contain 4,970 protein-coding genes and at least 450 non-coding RNAs.

In colloidal chemistry, flocculation is a process by which colloidal particles come out of suspension to sediment in the form of floc or flake, either spontaneously or due to the addition of a clarifying agent. The action differs from precipitation in that, prior to flocculation, colloids are merely suspended, under the form of a stable dispersion and are not truly dissolved in solution.

Concanavalin A (ConA) is a lectin originally extracted from the jack-bean. It is a member of the legume lectin family. It binds specifically to certain structures found in various sugars, glycoproteins, and glycolipids, mainly internal and nonreducing terminal α-D-mannosyl and α-D-glucosyl groups. Its physiological function in plants, however, is still unknown. ConA is a plant mitogen, and is known for its ability to stimulate mouse T-cell subsets giving rise to four functionally distinct T cell populations, including precursors to regulatory T cells; a subset of human suppressor T-cells is also sensitive to ConA. ConA was the first lectin to be available on a commercial basis, and is widely used in biology and biochemistry to characterize glycoproteins and other sugar-containing entities on the surface of various cells. It is also used to purify glycosylated macromolecules in lectin affinity chromatography, as well as to study immune regulation by various immune cells.

Saccharomyces is a genus of fungi that includes many species of yeasts. Saccharomyces is from Greek σάκχαρον (sugar) and μύκης (fungus) and means sugar fungus. Many members of this genus are considered very important in food production where they are known as brewer's yeast, baker's yeast and sourdough starter among others. They are unicellular and saprotrophic fungi. One example is Saccharomyces cerevisiae, which is used in making bread, wine, and beer, and for human and animal health. Other members of this genus include the wild yeast Saccharomyces paradoxus that is the closest relative to S. cerevisiae, Saccharomyces bayanus, used in making wine, and Saccharomyces cerevisiaevar. boulardii, used in medicine.
Pattern recognition receptors (PRRs) play a crucial role in the proper function of the innate immune system. PRRs are germline-encoded host sensors, which detect molecules typical for the pathogens. They are proteins expressed mainly by cells of the innate immune system, such as dendritic cells, macrophages, monocytes, neutrophils, as well as by epithelial cells, to identify two classes of molecules: pathogen-associated molecular patterns (PAMPs), which are associated with microbial pathogens, and damage-associated molecular patterns (DAMPs), which are associated with components of host's cells that are released during cell damage or death. They are also called primitive pattern recognition receptors because they evolved before other parts of the immune system, particularly before adaptive immunity. PRRs also mediate the initiation of antigen-specific adaptive immune response and release of inflammatory cytokines.

DC-SIGN also known as CD209 is a protein which in humans is encoded by the CD209 gene.
Rhamnose is a naturally occurring deoxy sugar. It can be classified as either a methyl-pentose or a 6-deoxy-hexose. Rhamnose predominantly occurs in nature in its L-form as L-rhamnose (6-deoxy-L-mannose). This is unusual, since most of the naturally occurring sugars are in D-form. Exceptions are the methyl pentoses L-fucose and L-rhamnose and the pentose L-arabinose. However, examples of naturally-occurring D-rhamnose include some species of bacteria, such as Pseudomonas aeruginosa and Helicobacter pylori.
The terms glycans and polysaccharides are defined by IUPAC as synonyms meaning "compounds consisting of a large number of monosaccharides linked glycosidically". However, in practice the term glycan may also be used to refer to the carbohydrate portion of a glycoconjugate, such as a glycoprotein, glycolipid, or a proteoglycan, even if the carbohydrate is only an oligosaccharide. Glycans usually consist solely of O-glycosidic linkages of monosaccharides. For example, cellulose is a glycan composed of β-1,4-linked D-glucose, and chitin is a glycan composed of β-1,4-linked N-acetyl-D-glucosamine. Glycans can be homo- or heteropolymers of monosaccharide residues, and can be linear or branched.

Langerin (CD207) is a type II transmembrane protein which is encoded by the CD207 gene in humans. It was discovered by scientists Sem Saeland and Jenny Valladeau as a main part of Birbeck granules. Langerin is C-type lectin receptor on Langerhans cells (LCs) and in mice also on dermal interstitial CD103+ dendritic cells (DC) and on resident CD8+ DC in lymph nodes.
The mannose receptor is a C-type lectin primarily present on the surface of macrophages, immature dendritic cells and liver sinusoidal endothelial cells, but is also expressed on the surface of skin cells such as human dermal fibroblasts and keratinocytes. It is the first member of a family of endocytic receptors that includes Endo180 (CD280), M-type PLA2R, and DEC-205 (CD205).
Anti-Saccharomyces cerevisiae antibodies (ASCAs) are antibodies against antigens presented by the cell wall of the yeast Saccharomyces cerevisiae. These antibodies are directed against oligomannose sequences α-1,3 Man n. ASCAs and perinuclear antineutrophil cytoplasmic antibodies (pANCAs) are the two most useful and often discriminating biomarkers for colitis. ASCA tends to recognize Crohn's disease more frequently, whereas pANCA tend to recognize ulcerative colitis.

Protein ERGIC-53 also known as ER-Golgi intermediate compartment 53 kDa protein or lectin mannose-binding 1 is a protein that in humans is encoded by the LMAN1 gene.
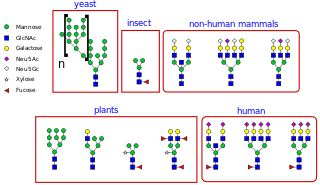
N-linked glycosylation is the attachment of an oligosaccharide, a carbohydrate consisting of several sugar molecules, sometimes also referred to as glycan, to a nitrogen atom, in a process called N-glycosylation, studied in biochemistry. The resulting protein is called an N-linked glycan, or simply an N-glycan.
BanLec is a lectin from the jacalin-related lectin family isolated from the fruit of the bananas Musa acuminata and Musa balbisiana. BanLec is one of the predominant proteins in the pulp of ripe bananas and has binding specificity for mannose and mannose-containing oligosaccharides. A 2010 study reported that BanLec was a potent inhibitor of HIV replication.

The role of yeast in winemaking is the most important element that distinguishes wine from fruit juice. In the absence of oxygen, yeast converts the sugars of the fruit into alcohol and carbon dioxide through the process of fermentation. The more sugars in the grapes, the higher the potential alcohol level of the wine if the yeast are allowed to carry out fermentation to dryness. Sometimes winemakers will stop fermentation early in order to leave some residual sugars and sweetness in the wine such as with dessert wines. This can be achieved by dropping fermentation temperatures to the point where the yeast are inactive, sterile filtering the wine to remove the yeast or fortification with brandy or neutral spirits to kill off the yeast cells. If fermentation is unintentionally stopped, such as when the yeasts become exhausted of available nutrients and the wine has not yet reached dryness, this is considered a stuck fermentation.
Glypiation is the addition by covalent bonding of a glycosylphosphatidylinositol (GPI) anchor and is a common post-translational modification that localizes proteins to cell membranes. This special kind of glycosylation is widely detected on surface glycoproteins in eukaryotes and some Archaea.

Grodziskie is a historical beer style from Poland made from oak-smoked wheat malt with a clear, light golden color, high carbonation, low alcohol content, low to moderate levels of hop bitterness, and a strong smoke flavor and aroma. The taste is light and crisp, with primary flavors coming from the smoked malt, the high mineral content of the water, and the strain of yeast used to ferment it. It was nicknamed "Polish Champagne" because of its high carbonation levels and valued as a high-quality beer for special occasions.
References
- ↑ (Lewin, 1984; Stratford, 1992)
- ↑ (Calleja, 1987)
- ↑ (Guinard and Lewis, 1993)
- ↑ (Guinard and Lewis, 1993)
- ↑ (Stewart et al., 1975; Stewart and Russell, 1986; Calleja, 1987; Speers et al., 1992; Jin and Speers, 1999)
- ↑ (Burns, 1937; Lindquist, 1953, Eddy, 1955; Masy et al., 1992
- ↑ (Burns, 1937; Lindquist, 1953)
- ↑ (Soares et al., 2004)
- ↑ (Calleja, 1987)
- ↑ (such as agitation, absence of sugars, a microamount of Ca2+, ethanol, etc.; Jin and Speers 1999)
- ↑ (see following)
- ↑ Speers, R.A., Wan, Y-Q., Jin, Y-L., and R. J. Stewart, R.J. 2006. Effects of fermentation parameters and cell wall properties on yeast flocculation. J. Inst. Brew. 112:246-254.
- ↑ (Miki et al., 1982) (figure 1.3)
- ↑ (section 1.5.4)
- ↑ (Speers, Smart, Stewart and Jin,1998)
- ↑ Speers, R.A., Smart, K., Stewart, R., Jin, Y-L., 1998. Zymolectins in Saccharomyces cerevisiae. Letter J. Inst. Brew., 104:298.
- ↑ (section 4.1)
- ↑ (El-Behhari et al., 2000)
- ↑ (Masy et al., 1992; Dengis and Rouxhet, 1997)
- ↑ (Jin and Speers, 1991, 1999)
- ↑ (Guo et al., 2000)
- ↑ (Burns, 1937; Miki et al., 1982; Nishihara and Toraya, 1987; Kihn et al., 1988; Stratford, 1989; Stratford and Assinder, 1991)
- ↑ (Miki, 1982; Stratford and Assinder, 1991; Masy et al., 1992; Smit et al., 1982; Stratford, 1993; Stratford and Carter, 1993; Teunissen et al., 1993; Teunissen et al., 1995a, b; Bony et al., 1997; Braley and Chaffin, 1999; Fleming and Pennings, 2001; He et al., 2002; Verstrepen et al., 2003) and is associated with the FLO1 gene (Watari, 1991 Masy et al., 1992; Stratford, 1993; Stratford and Carter, 1993; Teunissen et al., 1993; Teunissen et al., 1995a, b; Bony et al., 1997; Braley and Chaffin, 1999)
- ↑ (Stratford and Assinder, 1991; Masy, 1992)
- ↑ (Guo et al., 2000)
- ↑ (Stratford, 1989; Stratford and Assinder, 1991; Masy 1992)