Epoxy is the family of basic components or cured end products of epoxy resins. Epoxy resins, also known as polyepoxides, are a class of reactive prepolymers and polymers which contain epoxide groups. The epoxide functional group is also collectively called epoxy. The IUPAC name for an epoxide group is an oxirane.

Bisphenol A diglycidyl ether is an organic compound and is a liquid epoxy resin. The compound is a colorless viscous liquid. It is a key component of many epoxy resin formulations. Addition of further Bisphenol A and a catalyst and heat can produce Bisphenol A glycidyl ether epoxy resins of higher molecular weight that are solid.

Cyclohexanedimethanol (CHDM) is a mixture of isomeric organic compounds with formula C6H10(CH2OH)2. It is a colorless low-melting solid used in the production of polyester resins. Commercial samples consist of a mixture of cis and trans isomers. It is a di-substituted derivative of cyclohexane and is classified as a diol, meaning that it has two OH functional groups. Commercial CHDM typically has a cis/trans ratio of 30:70.
n-Butyl glycidyl ether is an industrial chemical used in adhesives, sealants, and as a paint or coating additive. It is principally used to reduce the viscosity of epoxy resin systems.

Reactive diluents are substances which reduce the viscosity of a lacquer or resin for processing and become part of the lacquer or coating during its subsequent curing via copolymerization. A non-reactive diluent would be a solvent or plasticizer.
2-Ethylhexyl glycidyl ether is a liquid organic molecule with formula C11H22O2 an industrial chemical used to reduce the viscosity of epoxy resins. These are then used in adhesives, sealants, and paints or coatings. It has the CAS Registry Number of 2461-15-6. It has the IUPAC name of 2-(2-ethylhexoxymethyl)oxirane. It also finds use in other polymer based applications.

o-Cresyl glycidyl ether (ortho-cresyl glycidyl ether, o-CGE) is a liquid aromatic organic chemical compound and chemically a glycidyl ether. It has the formula C10H12O2 and the CAS Registry Number 2210-79-9. It is one of a number of glycidyl ethers available commercially that are used to reduce the viscosity of epoxy resins. These are then further used in coatings, sealants, adhesives and elastomers.
Neopentyl glycol diglycidyl ether (NPGDGE) is an organic chemical in the glycidyl ether family. It is aliphatic and a colorless liquid. It has the formula C11H20O4 and the CAS registry number of 17557-23-2. It has two oxirane groups per molecule. Its principle use is in modifying epoxy resins.
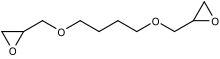
1,6-Hexanediol diglycidyl ether is an organic chemical in the glycidyl ether family. It is an aliphatic compound that is a colorless liquid. It has two epoxide (oxirane) groups per molecule. Its main use is in modifying epoxy resins especially viscosity reduction whilst flexibilizing. It is REACH registered.
1,4-Cyclohexanedimethanol diglycidyl ether is an organic chemical in the glycidyl ether family. It has the formula C14H24O4 and the IUPAC name is 2-[[4-(oxiran-2-ylmethoxymethyl)cyclohexyl]methoxymethyl]oxirane, and the CAS number 14228-73-0. It is It is REACH registered in Europe. It is an industrial chemical and a key use is in the reduction of viscosity of epoxy resin systems functioning as a reactive diluent.
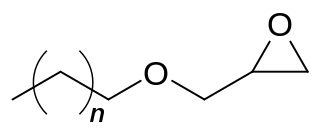
C12-C14 alcohol glycidyl ether (AGE) is an organic chemical in the glycidyl ether family. It is a mixture of mainly 12 and 14 carbon chain alcohols, also called fatty alcohols that have been glycidated. It is an industrial chemical used as a surfactant but primarily for epoxy resin viscosity reduction. It has the CAS number 68609-97-2 but the IUPAC name is more complex as it is a mixture and is 2-(dodecoxymethyl)oxirane;2-(tetradecoxymethyl)oxirane;2-(tridecoxymethyl)oxirane. Other names include dodecyl and tetradecyl glycidyl ethers and alkyl (C12-C14) glycidyl ether.

Trimethylolpropane triglycidyl ether (TMPTGE) is an organic chemical in the glycidyl ether family. It has the formula C15H26O6 and the IUPAC name is 2-[2,2-bis(oxiran-2-ylmethoxymethyl)butoxymethyl]oxirane, and the CAS number 3454-29-3. It also has another CAS number of 30499-70-8 A key use is as a modifier for epoxy resins as a reactive diluent.

Castor oil glycidyl ether is a liquid organic chemical in the glycidyl ether family. It is sometimes called castor oil triglycidyl ether. It has the theoretical formula C66H116O12 and the CAS number 14228-73-0. The IUPAC name is 2,3-bis[12-(oxiran-2-ylmethoxy)octadec-9-enoyloxy]propyl 12-(oxiran-2-ylmethoxy)octadec-9-enoate. A key use is acting as a modifier for epoxy resins as a reactive diluent that adds flexibility and improved mechanical properties.

C12-C13 alcohol glycidyl ether is a mixture of organic chemicals in the glycidyl ether family. It is a mixture of mainly 12 and 13 carbon chain alcohols, also called fatty alcohols that have been glycidated. It is an industrial chemical used as a surfactant but primarily for epoxy resin viscosity reduction. It has the CAS number 120547-52-6.

Trimethylolethane triglycidyl ether (TMETGE) is an organic chemical in the glycidyl ether family. It has the formula C14H24O6 and the IUPAC name is 2-({2-methyl-3-[(oxiran-2-yl)methoxy]-2-{[(oxiran-2-yl)methoxy]methyl}propoxy}methyl)oxirane. The CAS number is 68460-21-9. A key use is as a modifier for epoxy resins as a reactive diluent.

Poly(propylene glycol) diglycidyl ether (PPGDGE) is an organic chemical in the glycidyl ether family. There are a number of variations depending on the starting molecular weight of the polypropylene glycol. They have the formula (C3H6O)n.C6H10O3 and the IUPAC name is Poly[oxy(methyl-1,2-ethanediyl)],a-(2-oxiranylmethyl)-w-(2-oxiranylmethoxy)- A key use is as a modifier for epoxy resins as a reactive diluent and flexibilizer. It is REACH registered.
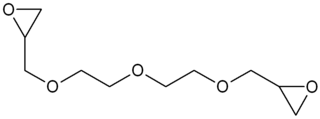
Diethylene glycol diglycidyl ether (DEGDGE) is an organic chemical in the glycidyl ether family with the formula C10H18O5.. The oxirane functionality makes it useful as a reactive diluent for epoxy resin viscosity reduction.

Diglycidyl resorcinol ether, also called Resorcinol diglycidyl ether (RDGE) is a liquid aromatic organic chemical compound and chemically a glycidyl ether.

Phenyl glycidyl ether, is a liquid aromatic organic chemical in the glycidyl ether class of compounds. It has the formula C9H10O2. It has the CAS Registry Number 122-60-1 and the IUPAC name of 2-(phenoxymethyl)oxirane. A key use is in the viscosity reduction of epoxy resin systems. It is REACH registered and on EINECS under the name 2,3-epoxypropyl phenyl ether.
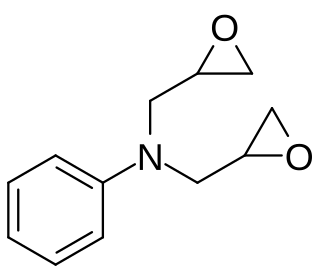
Diglycidyl aniline is an aromatic organic chemical in the glycidyl compound family. It is used to reduce the viscosity of epoxy resin systems. It has the empirical formula C12H15NO2 and the IUPAC name is N,N-bis(oxiran-2-ylmethyl)aniline. The CAS number is 2095-06-9. It is REACH registered in Europe with the EC number 218-259-5. A key use is in the viscosity reduction of epoxy resin systems functioning as a reactive diluent.