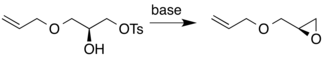
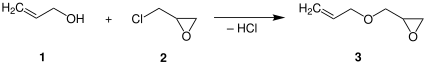The Sharpless epoxidation reaction is an enantioselective chemical reaction to prepare 2,3-epoxyalcohols from primary and secondary allylic alcohols. The oxidizing agent is tert-butyl hydroperoxide. The method relies on a catalyst formed from titanium tetra(isopropoxide) and diethyl tartrate.

Epoxy is the family of basic components or cured end products of epoxy resins. Epoxy resins, also known as polyepoxides, are a class of reactive prepolymers and polymers which contain epoxide groups. The epoxide functional group is also collectively called epoxy. The IUPAC name for an epoxide group is an oxirane.

In organic chemistry, an epoxide is a cyclic ether, where the ether forms a three-atom ring: two atoms of carbon and one atom of oxygen. This triangular structure has substantial ring strain, making epoxides highly reactive, more so than other ethers. They are produced on a large scale for many applications. In general, low molecular weight epoxides are colourless and nonpolar, and often volatile.
A diol is a chemical compound containing two hydroxyl groups. An aliphatic diol is also called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified.

Allyl alcohol is an organic compound with the structural formula CH2=CHCH2OH. Like many alcohols, it is a water-soluble, colourless liquid. It is more toxic than typical small alcohols. Allyl alcohol is used as a precursor to many specialized compounds such as flame-resistant materials, drying oils, and plasticizers. Allyl alcohol is the smallest representative of the allylic alcohols.

The Jacobsen epoxidation, sometimes also referred to as Jacobsen-Katsuki epoxidation is a chemical reaction which allows enantioselective epoxidation of unfunctionalized alkyl- and aryl- substituted alkenes. It is complementary to the Sharpless epoxidation (used to form epoxides from the double bond in allylic alcohols). The Jacobsen epoxidation gains its stereoselectivity from a C2 symmetric manganese(III) salen-like ligand, which is used in catalytic amounts. The manganese atom transfers an oxygen atom from chlorine bleach or similar oxidant. The reaction takes its name from its inventor, Eric Jacobsen, with Tsutomu Katsuki sometimes being included. Chiral-directing catalysts are useful to organic chemists trying to control the stereochemistry of biologically active compounds and develop enantiopure drugs.

The Johnson–Corey–Chaykovsky reaction is a chemical reaction used in organic chemistry for the synthesis of epoxides, aziridines, and cyclopropanes. It was discovered in 1961 by A. William Johnson and developed significantly by E. J. Corey and Michael Chaykovsky. The reaction involves addition of a sulfur ylide to a ketone, aldehyde, imine, or enone to produce the corresponding 3-membered ring. The reaction is diastereoselective favoring trans substitution in the product regardless of the initial stereochemistry. The synthesis of epoxides via this method serves as an important retrosynthetic alternative to the traditional epoxidation reactions of olefins.
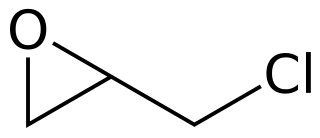
Epichlorohydrin is an organochlorine compound and an epoxide. Despite its name, it is not a halohydrin. It is a colorless liquid with a pungent, garlic-like odor, moderately soluble in water, but miscible with most polar organic solvents. It is a chiral molecule generally existing as a racemic mixture of right-handed and left-handed enantiomers. Epichlorohydrin is a highly reactive electrophilic compound and is used in the production of glycerol, plastics, epoxy glues and resins, epoxy diluents and elastomers.
In organic chemistry, kinetic resolution is a means of differentiating two enantiomers in a racemic mixture. In kinetic resolution, two enantiomers react with different reaction rates in a chemical reaction with a chiral catalyst or reagent, resulting in an enantioenriched sample of the less reactive enantiomer. As opposed to chiral resolution, kinetic resolution does not rely on different physical properties of diastereomeric products, but rather on the different chemical properties of the racemic starting materials. The enantiomeric excess (ee) of the unreacted starting material continually rises as more product is formed, reaching 100% just before full completion of the reaction. Kinetic resolution relies upon differences in reactivity between enantiomers or enantiomeric complexes.

Glycidol is an organic compound that contains both epoxide and alcohol functional groups. Being bifunctional, it has a variety of industrial uses. The compound is a slightly viscous liquid that is slightly unstable and is not often encountered in pure form.

Jacobsen's catalyst is the common name for N,N'-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediaminomanganese(III) chloride, a coordination compound of manganese and a salen-type ligand. It is used as an asymmetric catalyst in the Jacobsen epoxidation, which is renowned for its ability to enantioselectively transform prochiral alkenes into epoxides. Before its development, catalysts for the asymmetric epoxidation of alkenes required the substrate to have a directing functional group, such as an alcohol as seen in the Sharpless epoxidation. This compound has two enantiomers, which give the appropriate epoxide product from the alkene starting material.
2-Ethylhexyl glycidyl ether is a liquid organic molecule with formula C11H22O2 an industrial chemical used to reduce the viscosity of epoxy resins. These are then used in adhesives, sealants, and paints or coatings. It has the CAS Registry Number of 2461-15-6. It has the IUPAC name of 2-(2-ethylhexoxymethyl)oxirane. It also finds use in other polymer based applications.

o-Cresyl glycidyl ether (ortho-cresyl glycidyl ether, o-CGE) is a liquid aromatic organic chemical compound and chemically a glycidyl ether. It has the formula C10H12O2 and the CAS Registry Number 2210-79-9. It is one of a number of glycidyl ethers available commercially that are used to reduce the viscosity of epoxy resins. These are then further used in coatings, sealants, adhesives and elastomers.
Neopentyl glycol diglycidyl ether (NPGDGE) is an organic chemical in the glycidyl ether family. It is aliphatic and a colorless liquid. It has the formula C11H20O4 and the CAS registry number of 17557-23-2. It has two oxirane groups per molecule. Its principle use is in modifying epoxy resins.
1,6-Hexanediol diglycidyl ether is an organic chemical in the glycidyl ether family. It is an aliphatic compound that is a colorless liquid. It has two epoxide (oxirane) groups per molecule. Its main use is in modifying epoxy resins especially viscosity reduction whilst flexibilizing. It is REACH registered.
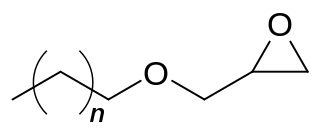
C12-C14 alcohol glycidyl ether (AGE) is an organic chemical in the glycidyl ether family. It is a mixture of mainly 12 and 14 carbon chain alcohols, also called fatty alcohols that have been glycidated. It is an industrial chemical used as a surfactant but primarily for epoxy resin viscosity reduction. It has the CAS number 68609-97-2 but the IUPAC name is more complex as it is a mixture and is 2-(dodecoxymethyl)oxirane;2-(tetradecoxymethyl)oxirane;2-(tridecoxymethyl)oxirane. Other names include dodecyl and tetradecyl glycidyl ethers and alkyl (C12-C14) glycidyl ether.

Castor oil glycidyl ether is a liquid organic chemical in the glycidyl ether family. It is sometimes called castor oil triglycidyl ether. It has the theoretical formula C66H116O12 and the CAS number 14228-73-0. The IUPAC name is 2,3-bis[12-(oxiran-2-ylmethoxy)octadec-9-enoyloxy]propyl 12-(oxiran-2-ylmethoxy)octadec-9-enoate. A key use is acting as a modifier for epoxy resins as a reactive diluent that adds flexibility and improved mechanical properties.

C12-C13 alcohol glycidyl ether is a mixture of organic chemicals in the glycidyl ether family. It is a mixture of mainly 12 and 13 carbon chain alcohols, also called fatty alcohols that have been glycidated. It is an industrial chemical used as a surfactant but primarily for epoxy resin viscosity reduction. It has the CAS number 120547-52-6.
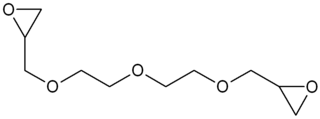
Diethylene glycol diglycidyl ether (DEGDGE) is an organic chemical in the glycidyl ether family with the formula C10H18O5.. The oxirane functionality makes it useful as a reactive diluent for epoxy resin viscosity reduction.

Phenyl glycidyl ether, is a liquid aromatic organic chemical in the glycidyl ether class of compounds. It has the formula C9H10O2. It has the CAS Registry Number 122-60-1 and the IUPAC name of 2-(phenoxymethyl)oxirane. A key use is in the viscosity reduction of epoxy resin systems. It is REACH registered and on EINECS under the name 2,3-epoxypropyl phenyl ether.