Related Research Articles

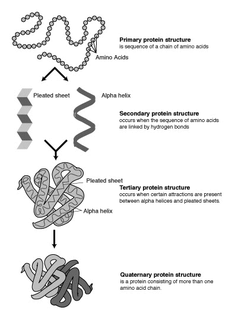
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity.

Protein secondary structure is the three dimensional form of local segments of proteins. The two most common secondary structural elements are alpha helices and beta sheets, though beta turns and omega loops occur as well. Secondary structure elements typically spontaneously form as an intermediate before the protein folds into its three dimensional tertiary structure.

The soybean, soy bean, or soya bean is a species of legume native to East Asia, widely grown for its edible bean, which has numerous uses.
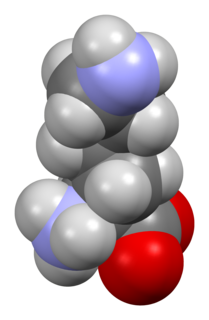
Lysine (symbol Lys or K) is an α-amino acid that is a precursor to many proteins. It contains an α-amino group (which is in the protonated −NH3+ form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain lysyl ((CH2)4NH2), classifying it as a basic, charged (at physiological pH), aliphatic amino acid. It is encoded by the codons AAA and AAG. Like almost all other amino acids, the α-carbon is chiral and lysine may refer to either enantiomer or a racemic mixture of both. For the purpose of this article, lysine will refer to the biologically active enantiomer L-lysine, where the α-carbon is in the S configuration.

Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large proportion of all proteins are part of this category. For instance, at least 1000 human proteins contain zinc-binding protein domains although there may be up to 3000 human zinc metalloproteins.
Protein structure prediction is the inference of the three-dimensional structure of a protein from its amino acid sequence—that is, the prediction of its secondary and tertiary structure from primary structure. Structure prediction is different from the inverse problem of protein design. Protein structure prediction is one of the most important goals pursued by computational biology; and it is important in medicine and biotechnology.
In a chain-like biological molecule, such as a protein or nucleic acid, a structural motif is a common three-dimensional structure that appears in a variety of different, evolutionarily unrelated molecules. A structural motif does not have to be associated with a sequence motif; it can be represented by different and completely unrelated sequences in different proteins or RNA.

Thyroxine-binding globulin (TBG) is a globulin protein that in humans is encoded by the SERPINA7 gene. TBG binds thyroid hormones in circulation. It is one of three transport proteins (along with transthyretin and serum albumin) responsible for carrying the thyroid hormones thyroxine (T4) and triiodothyronine (T3) in the bloodstream. Of these three proteins, TBG has the highest affinity for T4 and T3 but is present in the lowest concentration relative to transthyretin and albumin, which also bind T3 and T4 in circulation. Despite its low concentration, TBG carries the majority of T4 in the blood plasma. Due to the very low concentration of T4 and T3 in the blood, TBG is rarely more than 25% saturated with its ligand. Unlike transthyretin and albumin, TBG has a single binding site for T4/T3. TBG is synthesized primarily in the liver as a 54-kDa protein. In terms of genomics, TBG is a serpin; however, it has no inhibitory function like many other members of this class of proteins.

Transcortin, also known as corticosteroid-binding globulin (CBG) or serpin A6, is a protein produced in the liver in animals. In humans it is encoded by the SERPINA6 gene. It is an alpha-globulin.

Protein structure is the three-dimensional arrangement of atoms in an amino acid-chain molecule. Proteins are polymers – specifically polypeptides – formed from sequences of amino acids, the monomers of the polymer. A single amino acid monomer may also be called a residue indicating a repeating unit of a polymer. Proteins form by amino acids undergoing condensation reactions, in which the amino acids lose one water molecule per reaction in order to attach to one another with a peptide bond. By convention, a chain under 30 amino acids is often identified as a peptide, rather than a protein. To be able to perform their biological function, proteins fold into one or more specific spatial conformations driven by a number of non-covalent interactions such as hydrogen bonding, ionic interactions, Van der Waals forces, and hydrophobic packing. To understand the functions of proteins at a molecular level, it is often necessary to determine their three-dimensional structure. This is the topic of the scientific field of structural biology, which employs techniques such as X-ray crystallography, NMR spectroscopy, cryo electron microscopy (cryo-EM) and dual polarisation interferometry to determine the structure of proteins.

Titin is a protein that in humans is encoded by the TTN gene. Titin is a giant protein, greater than 1 µm in length, that functions as a molecular spring which is responsible for the passive elasticity of muscle. It comprises 244 individually folded protein domains connected by unstructured peptide sequences. These domains unfold when the protein is stretched and refold when the tension is removed.

Sex hormone-binding globulin (SHBG) or sex steroid-binding globulin (SSBG) is a glycoprotein that binds to androgens and estrogens. Other steroid hormones such as progesterone, cortisol, and other corticosteroids are bound by transcortin. SHBG is found in all vertebrates apart from birds.

A catalytic triad is a set of three coordinated amino acids that can be found in the active site of some enzymes. Catalytic triads are most commonly found in hydrolase and transferase enzymes. An acid-base-nucleophile triad is a common motif for generating a nucleophilic residue for covalent catalysis. The residues form a charge-relay network to polarise and activate the nucleophile, which attacks the substrate, forming a covalent intermediate which is then hydrolysed to release the product and regenerate free enzyme. The nucleophile is most commonly a serine or cysteine amino acid, but occasionally threonine or even selenocysteine. The 3D structure of the enzyme brings together the triad residues in a precise orientation, even though they may be far apart in the sequence.
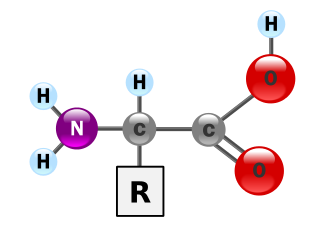
Proteins are essential nutrients for the human body. They are one of the building blocks of body tissue and can also serve as a fuel source. As a fuel, proteins provide as much energy density as carbohydrates: 4 kcal per gram; in contrast, lipids provide 9 kcal per gram. The most important aspect and defining characteristic of protein from a nutritional standpoint is its amino acid composition.

An isopeptide bond is an amide bond that can form for example between a carboxyl group of one amino acid and an amino group of another. At least one of these joining groups is part of the side chain of one of these amino acids, unlike in a peptide bond which is sometimes called a eupeptide bond, especially when discussing about both of these bond types in the same context to make a distinction between the two.

Soy protein is a protein that is isolated from soybean. It is made from soybean meal that has been dehulled and defatted. Dehulled and defatted soybeans are processed into three kinds of high protein commercial products: soy flour, concentrates, and isolates. Soy protein isolate has been used since 1959 in foods for its functional properties.

Acylamino-acid-releasing enzyme is an enzyme that in humans is encoded by the APEH gene.

The cupin superfamily is a diverse superfamily of proteins named after its conserved barrel domain. The superfamily includes a wide variety of enzymes as well as non-enzymatic seed storage proteins.

Pea protein is a food product and protein supplement derived and extracted from yellow and green split peas, Pisum sativum. It can be used as a supplement to increase an individual's protein or other nutrient intake, or as a substitute for other food products. It is also used as a functional ingredient in food-manufacturing, such as a thickener, foaming agent, or an emulsifier.
Protein quality is the digestibility and quantity of essential amino acids for providing the proteins in correct ratios for human consumption. There are various methods that rank the quality of different types of protein, some of which are outdated and no longer in use, or not considered as useful as they once were thought to be. The Protein Digestibility Corrected Amino Acid Score (PDCAAS), which was recommended by the Food and Agriculture Organization of the United Nations (FAO), became the industry standard in 1993. FAO has recently recommended the newer Digestible Indispensable Amino Acid Score (DIAAS) to supersede PDCAAS. The dairy industry is in favor of this, because while PDCAAS truncates all protein types that exceed the essential amino acid (EAA) requirements to 1.0, DIAAS allows a higher than 1.0 ranking: while for example both soy protein isolate and whey isolate are ranked 1.0 according to PDCAAS, in the DIAAS system, whey has a higher score than soy.
References
- 1 2 "Pulse Proteins" (PDF). Pulsecanada.com. Archived from the original (PDF) on 2017-03-05. Retrieved 2017-02-26.
- ↑ Michael C. Lawrence (1999). "Structural Relationships of 7S and 11S Globulins". Structural Relationships of 7S and 11S Globulins - Springer. pp. 517–541. doi:10.1007/978-94-011-4431-5_22. ISBN 978-94-010-5904-6.
- ↑ Motoyasu Adachi; Jiro Kanamori; Taro Masuda; Kazuhiro Yagasaki; Keisuke Kitamura; Bunzo Mikami; Shigeru Utsumi (2003). "Crystal structure of soybean 11S globulin : Glycinin A3B4 homohexamer". Proceedings of the National Academy of Sciences. 100 (12): 7395–7400. Bibcode:2003PNAS..100.7395A. doi: 10.1073/pnas.0832158100 . PMC 165886 . PMID 12771376.