Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity.

The enzyme-linked immunosorbent assay (ELISA) is a commonly used analytical biochemistry assay, first described by Eva Engvall and Peter Perlmann in 1971. The assay is a solid-phase type of enzyme immunoassay (EIA) to detect the presence of a ligand in a liquid sample using antibodies directed against the protein to be measured. ELISA has been used as a diagnostic tool in medicine, plant pathology, and biotechnology, as well as a quality control check in various industries.

Transcortin, also known as corticosteroid-binding globulin (CBG) or serpin A6, is a protein produced in the liver in animals. In humans it is encoded by the SERPINA6 gene. It is an alpha-globulin.
Haptens are small molecules that elicit an immune response only when attached to a large carrier such as a protein; the carrier may be one that also does not elicit an immune response by itself. The mechanisms of absence of immune response may vary and involve complex immunological interactions, but can include absent or insufficient co-stimulatory signals from antigen-presenting cells.

α2-Macroglobulin (α2M) or alpha-2-macroglobulin is a large plasma protein found in the blood. It is mainly produced by the liver, and also locally synthesized by macrophages, fibroblasts, and adrenocortical cells. In humans it is encoded by the A2M gene.
In biochemistry, biotinylation is the process of covalently attaching biotin to a protein, nucleic acid or other molecule. Biotinylation is rapid, specific and is unlikely to disturb the natural function of the molecule due to the small size of biotin. Biotin binds to streptavidin and avidin with an extremely high affinity, fast on-rate, and high specificity, and these interactions are exploited in many areas of biotechnology to isolate biotinylated molecules of interest. Biotin-binding to streptavidin and avidin is resistant to extremes of heat, pH and proteolysis, making capture of biotinylated molecules possible in a wide variety of environments. Also, multiple biotin molecules can be conjugated to a protein of interest, which allows binding of multiple streptavidin, avidin or neutravidin protein molecules and increases the sensitivity of detection of the protein of interest. There is a large number of biotinylation reagents available that exploit the wide range of possible labelling methods. Due to the strong affinity between biotin and streptavidin, the purification of biotinylated proteins has been a widely used approach to identify protein-protein interactions and post-translational events such as ubiquitylation in molecular biology.
The Bradford protein assay was developed by Marion M. Bradford in 1976. It is a quick and accurate spectroscopic analytical procedure used to measure the concentration of protein in a solution. The reaction is dependent on the amino acid composition of the measured proteins.

Glutathione S-transferases (GSTs), previously known as ligandins, are a family of eukaryotic and prokaryotic phase II metabolic isozymes best known for their ability to catalyze the conjugation of the reduced form of glutathione (GSH) to xenobiotic substrates for the purpose of detoxification. The GST family consists of three superfamilies: the cytosolic, mitochondrial, and microsomal—also known as MAPEG—proteins. Members of the GST superfamily are extremely diverse in amino acid sequence, and a large fraction of the sequences deposited in public databases are of unknown function. The Enzyme Function Initiative (EFI) is using GSTs as a model superfamily to identify new GST functions.
Affinity chromatography is a method of separating a biomolecule from a mixture, based on a highly specific macromolecular binding interaction between the biomolecule and another substance. The specific type of binding interaction depends on the biomolecule of interest; antigen and antibody, enzyme and substrate, receptor and ligand, or protein and nucleic acid binding interactions are frequently exploited for isolation of various biomolecules. Affinity chromatography is useful for its high selectivity and resolution of separation, compared to other chromatographic methods.
Serum albumin, often referred to simply as blood albumin, is an albumin found in vertebrate blood. Human serum albumin is encoded by the ALB gene. Other mammalian forms, such as bovine serum albumin, are chemically similar.

Whey protein is a mixture of proteins isolated from whey, the liquid material created as a by-product of cheese production. The proteins consist of α-lactalbumin, β-lactoglobulin, serum albumin and immunoglobulins. Glycomacropeptide also makes up the third largest component but is not a protein. Whey protein is commonly marketed as a protein supplement, and various health claims have been attributed to it. A review published in 2010 in the European Food Safety Authority Journal concluded that the provided literature did not adequately support the proposed claims.

Human serum albumin is the serum albumin found in human blood. It is the most abundant protein in human blood plasma; it constitutes about half of serum protein. It is produced in the liver. It is soluble in water, and it is monomeric.
Albumin is a family of globular proteins, the most common of which are the serum albumins. All of the proteins of the albumin family are water-soluble, moderately soluble in concentrated salt solutions, and experience heat denaturation. Albumins are commonly found in blood plasma and differ from other blood proteins in that they are not glycosylated. Substances containing albumins are called albuminoids.
Plasma protein binding refers to the degree to which medications attach to blood proteins within the blood plasma. A drug's efficacy may be affected by the degree to which it binds. The less bound a drug is, the more efficiently it can traverse or diffuse through cell membranes. Common blood proteins that drugs bind to are human serum albumin, lipoprotein, glycoprotein, and α, β‚ and γ globulins.
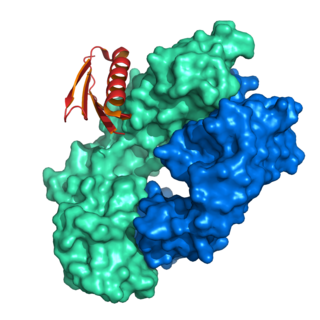
Protein L was first isolated from the surface of bacterial species Peptostreptococcus magnus and was found to bind immunoglobulins through L chain interaction, from which the name was suggested. It consists of 719 amino acid residues. The molecular weight of protein L purified from the cell walls of Peptostreptoccus magnus was first estimated as 95kD by SDS-PAGE in the presence of reducing agent 2-mercaptoethanol, while the molecular weight was determined to 76kD by gel chromatography in the presence of 6 M guanidine HCl. Protein L does not contain any interchain disulfide loops, nor does it consist of disulfide-linked subunits. It is an acidic molecule with a pI of 4.0. Unlike protein A and protein G, which bind to the Fc region of immunoglobulins (antibodies), protein L binds antibodies through light chain interactions. Since no part of the heavy chain is involved in the binding interaction, Protein L binds a wider range of antibody classes than protein A or G. Protein L binds to representatives of all antibody classes, including IgG, IgM, IgA, IgE and IgD. Single chain variable fragments (scFv) and Fab fragments also bind to protein L.

α-Parinaric acid is a conjugated polyunsaturated fatty acid. Discovered by Tsujimoto and Koyanagi in 1933, it contains 18 carbon atoms and 4 conjugated double bonds. The repeating single bond-double bond structure of α-parinaric acid distinguishes it structurally and chemically from the usual "methylene-interrupted" arrangement of polyunsaturated fatty acids that have double-bonds and single bonds separated by a methylene unit (−CH2−). Because of the fluorescent properties conferred by the alternating double bonds, α-parinaric acid is commonly used as a molecular probe in the study of biomembranes.

Vitamin D-binding protein (DBP), also/originally known as gc-globulin, is a protein that in humans is encoded by the GC gene. DBP is genetically the oldest member of the albuminoid family and appeared early in the evolution of vertebrates.

Disuccinimidyl suberate (DSS) is a six-carbon lysine-reactive non-cleavable cross-linking agent.
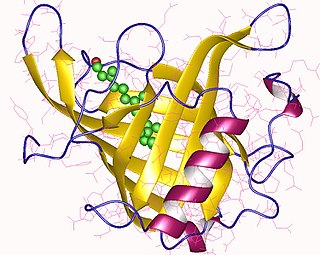
Retinol-binding proteins (RBP) are a family of proteins with diverse functions. They are carrier proteins that bind retinol. Assessment of retinol-binding protein is used to determine visceral protein mass in health-related nutritional studies.
Peptide therapeutics are peptides or polypeptides which are used to for the treatment of diseases. Naturally occurring peptides may serve as hormones, growth factors, neurotransmitters, ion channel ligands, and anti-infectives; peptide therapeutics mimic such functions. Peptide Therapeutics are seen as relatively safe and well-tolerated as peptides can be metabolized by the body.