Pyrrole is a heterocyclic, aromatic, organic compound, a five-membered ring with the formula C4H4NH. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., N-methylpyrrole, C4H4NCH3. Porphobilinogen, a trisubstituted pyrrole, is the biosynthetic precursor to many natural products such as heme.
The Friedel–Crafts reactions are a set of reactions developed by Charles Friedel and James Crafts in 1877 to attach substituents to an aromatic ring. Friedel–Crafts reactions are of two main types: alkylation reactions and acylation reactions. Both proceed by electrophilic aromatic substitution.

A dipeptide is an organic compound derived from two amino acids. The constituent amino acids can be the same or different. When different, two isomers of the dipeptide are possible, depending on the sequence. Several dipeptides are physiologically important, and some are both physiologically and commercially significant. A well known dipeptide is aspartame, an artificial sweetener.

In stereochemistry, a chiral auxiliary is a stereogenic group or unit that is temporarily incorporated into an organic compound in order to control the stereochemical outcome of the synthesis. The chirality present in the auxiliary can bias the stereoselectivity of one or more subsequent reactions. The auxiliary can then be typically recovered for future use.

The Robinson–Gabriel synthesis is an organic reaction in which a 2-acylamino-ketone reacts intramolecularly followed by a dehydration to give an oxazole. A cyclodehydrating agent is needed to catalyze the reaction It is named after Sir Robert Robinson and Siegmund Gabriel who described the reaction in 1909 and 1910, respectively.

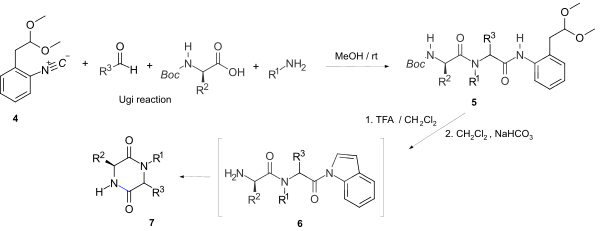
Cyclic peptides are polypeptide chains which contain a circular sequence of bonds. This can be through a connection between the amino and carboxyl ends of the peptide, for example in cyclosporin; a connection between the amino end and a side chain, for example in bacitracin; the carboxyl end and a side chain, for example in colistin; or two side chains or more complicated arrangements, for example in alpha-amanitin. Many cyclic peptides have been discovered in nature and many others have been synthesized in the laboratory. Their length ranges from just two amino acid residues to hundreds. In nature they are frequently antimicrobial or toxic; in medicine they have various applications, for example as antibiotics and immunosuppressive agents. Thin-Layer Chromatography (TLC) is a convenient method to detect cyclic peptides in crude extract from bio-mass.

Pseudoproline derivatives are artificially created dipeptides to minimize aggregation during Fmoc solid-phase synthesis of peptides.

The Erlenmeyer–Plöchl azlactone and amino acid synthesis, named after Friedrich Gustav Carl Emil Erlenmeyer who partly discovered the reaction, is a series of chemical reactions which transform an N-acyl glycine to various other amino acids via an oxazolone.

Spirotryprostatin B is an indolic alkaloid found in the Aspergillus fumigatus fungus that belongs to a class of naturally occurring 2,5-diketopiperazines. Spirotryprostatin B and several other indolic alkaloids have been found to have anti-mitotic properties, and as such they have become of great interest as anti-cancer drugs. Because of this, the total syntheses of these compounds is a major pursuit of organic chemists, and a number of different syntheses have been published in the chemical literature.
The Schöllkopf method or Schöllkopf Bis-Lactim Amino Acid Synthesis is a method in organic chemistry for the asymmetric synthesis of chiral amino acids. The method was established in 1981 by Ulrich Schöllkopf. In it glycine is a substrate, valine a chiral auxiliary and the reaction taking place an alkylation.

Epelsiban is an orally bioavailable drug which acts as a selective and potent oxytocin receptor antagonist. It was initially developed by GlaxoSmithKline (GSK) for the treatment of premature ejaculation in men and then as an agent to enhance embryo or blastocyst implantation in women undergoing embryo or blastocyst transfer associated with in vitro fertilization (IVF)., and was also investigated for use in the treatment of adenomyosis.

The Enders SAMP/RAMP hydrazone alkylation reaction is an asymmetric carbon-carbon bond formation reaction facilitated by pyrrolidine chiral auxiliaries. It was pioneered by E. J. Corey and D. Enders in 1976, and was further developed by D. Enders and his group. This method is usually a three-step sequence. The first step is to form the hydrazone between (S)-1-amino-2-methoxymethylpyrrolidine (SAMP) or (R)-1-amino-2-methoxymethylpyrrolidine (RAMP) and a ketone or aldehyde. Afterwards, the hydrazone is deprotonated by lithium diisopropylamide (LDA) to form an azaenolate, which reacts with alkyl halides or other suitable electrophiles to give alkylated hydrazone species with the simultaneous generation of a new chiral center. Finally, the alkylated ketone or aldehyde can be regenerated by ozonolysis or hydrolysis.
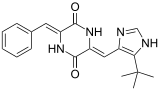
Retosiban also known as GSK-221,149-A is an oral drug which acts as an oxytocin receptor antagonist. It is being developed by GlaxoSmithKline for the treatment of preterm labour. Retosiban has high affinity for the oxytocin receptor and has greater than 1400-fold selectivity over the related vasopressin receptors
In organic chemistry, the Fráter–Seebach alkylation is a diastereoselective alkylation of chiral beta-hydroxy esters using strong bases. The reaction was first published by Georg Fráter in 1979; in 1980, Dieter Seebach reported about a similar reaction with malic acid ester.

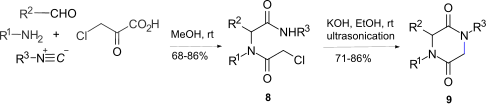
A diketopiperazine (DKP), also known as a dioxopiperazine or piperazinedione, is a class of organic compounds related to piperazine but containing two amide linkages. DKP's are the smallest known class of cyclic peptide. Despite their name, they are not ketones, but amides. Three regioisomers are possible, differing in the locations of the carbonyl groups.

Fumitremorgins are tremorogenic metabolites of Aspergillus and Penicillium, that belong to a class of naturally occurring 2,5-diketopiperazines.

Verruculogen is a mycotoxin produced by certain strains of aspergillus that belongs to a class of naturally occurring 2,5-diketopiperazines. It is an annulated analogue of cyclo(L-Trp-L-Pro) which belongs to the most abundant and structurally diverse class of tryptophan-proline 2,5-diketopiperazine natural products. It produces tremors in mice due to its neurotoxic properties. It also tested positive in a Salmonella/mammalian microsome assay and was shown to be genotoxic. It is a potent blocker of calcium-activated potassium channels.

Bottromycin is a macrocyclic peptide with antibiotic activity. It was first discovered in 1957 as a natural product isolated from Streptomyces bottropensis. It has been shown to inhibit methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococci (VRE) among other Gram-positive bacteria and mycoplasma. Bottromycin is structurally distinct from both vancomycin, a glycopeptide antibiotic, and methicillin, a beta-lactam antibiotic.

Brevianamide F , also known as cyclo-(L-Trp-L-Pro), belongs to a class of naturally occurring 2,5-diketopiperazines. It is the simplest member and the biosynthetic precursor of a large family of biologically active prenylated tryptophan-proline 2,5-diketopiperazines that are produced by the fungi A. fumigatus and Aspergillus sp. It has been isolated from the bacterium Streptomyces sp. strain TN58 and shown to possess activity against the Gram-positive bacteria S. aureus and Micrococcus luteus. It has also been isolated from Bacillus cereus associated with the entomopathogenic nematode Rhabditis (Oscheius) sp. and shown to have antifungal activity against T. rubrum, C. neoformans, and C. albicans, better than amphotericin B. Although the proline 2,5-diketopiperazines are the most abundant and structurally diverse 2,5-diketopiperazines found in food, cyclo(L-Trp-L-Pro) has only been found as a minor 2,5-diketopiperazine (8.2 ppm) in autolyzed yeast extract. Initially, cyclo(L-Trp-L-Pro) and its DL, LD, and DD isomers showed potential for use in the treatment of cardiovascular dysfunction, but they were later shown to be hepatotoxic.
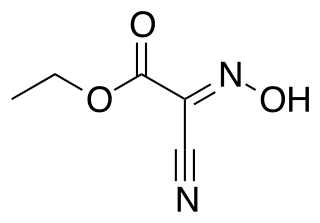
Ethyl cyanohydroxyiminoacetate (oxyma) is the oxime of ethyl cyanoacetate and finds use as an additive for carbodiimides, such as dicyclohexylcarbodiimide (DCC) in peptide synthesis. It acts as a neutralizing reagent for the basicity or nucleophilicity of the DCC due to its pronounced acidity and suppresses base catalyzed side reactions, in particular racemization.