In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl functional group bound to a saturated carbon atom. Alcohols range from the simple, like methanol and ethanol, to complex, like sucrose and cholesterol. The presence of an OH group strongly modifies the properties of hydrocarbons, conferring hydrophilic (water-loving) properties. The OH group provides a site at which many reactions can occur.

Ethanol is an organic compound with the chemical formula CH3CH2OH. It is an alcohol, with its formula also written as C2H5OH, C2H6O or EtOH, where Et stands for ethyl. Ethanol is a volatile, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. It is a psychoactive recreational drug, and the active ingredient in alcoholic drinks.

Diethyl malonate, also known as DEM, is the diethyl ester of malonic acid. It occurs naturally in grapes and strawberries as a colourless liquid with an apple-like odour, and is used in perfumes. It is also used to synthesize other compounds such as barbiturates, artificial flavourings, vitamin B1, and vitamin B6.

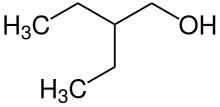
Butan-2-ol, or sec-butanol, is an organic compound with formula CH3CH(OH)CH2CH3. Its structural isomers are 1-butanol, isobutanol, and tert-butanol. 2-Butanol is chiral and thus can be obtained as either of two stereoisomers designated as (R)-(−)-butan-2-ol and (S)-(+)-butan-2-ol. It is normally encountered as a 1:1 mixture of the two stereoisomers — a racemic mixture.

tert-Butyl alcohol is the simplest tertiary alcohol, with a formula of (CH3)3COH (sometimes represented as t-BuOH). Its isomers are 1-butanol, isobutanol, and butan-2-ol. tert-Butyl alcohol is a colorless solid, which melts near room temperature and has a camphor-like odor. It is miscible with water, ethanol and diethyl ether.
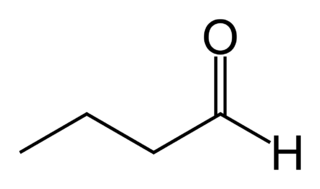
Butyraldehyde, also known as butanal, is an organic compound with the formula CH3(CH2)2CHO. This compound is the aldehyde derivative of butane. It is a colorless flammable liquid with an unpleasant smell. It is miscible with most organic solvents.

Phosphoryl chloride is a colourless liquid with the formula POCl3. It hydrolyses in moist air releasing phosphoric acid and fumes of hydrogen chloride. It is manufactured industrially on a large scale from phosphorus trichloride and oxygen or phosphorus pentoxide. It is mainly used to make phosphate esters such as tricresyl phosphate.

n-Butyl acetate is an organic compound with the formula CH3CO2(CH2)3CH3. A colorless, flammable liquid, it is the ester derived from n-butanol and acetic acid. It is found in many types of fruit, where it imparts characteristic flavors and has a sweet smell of banana or apple. It is used as an industrial solvent.
Hexachlorobenzene, or perchlorobenzene, is an organochloride with the molecular formula C6Cl6. It is a fungicide formerly used as a seed treatment, especially on wheat to control the fungal disease bunt. It has been banned globally under the Stockholm Convention on Persistent Organic Pollutants.

Miscibility is the property of two substances to mix in all proportions, forming a homogeneous mixture. The term is most often applied to liquids but also applies to solids and gases. An example in liquids is the miscibility of water and ethanol as they mix in all proportions.

tert-Amyl alcohol (TAA) or 2-methylbutan-2-ol (2M2B), is a branched pentanol.

2-Methyl-1-butanol (IUPAC name, also called active amyl alcohol) is an organic compound with the formula CH3CH2CH(CH3)CH2OH. It is one of several isomers of amyl alcohol. A colorless liquid, it occurs naturally in trace amounts and has attracted some attention as a potential biofuel, exploiting its hydrophobic (gasoline-like) and branched structure. It is chiral.

2-Pentanol is an organic chemical compound. It is used as a solvent and an intermediate in the manufacturing of other chemicals. 2-Pentanol is a component of many mixtures of amyl alcohols sold industrially. 2-Pentanol is chiral and thus can be obtained as either of two stereoisomers designated as (R)-(−)-2-pentanol and (S)-(+)-2-pentanol.

3-Hexanol is an organic chemical compound. It occurs naturally in the flavor and aroma of plants such as pineapple and is used as a food additive to add flavor.

2-Methyl-2-pentanol is an organic chemical compound. It can be added to a gas chromatograph to help distinguish between branched compounds, especially alcohols. Its presence in urine can be used to test for exposure to 2-methylpentane. As with many other short-chain alcohols, 2-methyl-2-pentanol can produce intoxication and sedative effects similar to those of ethanol, though it is more irritating to mucous membranes and generally more toxic to the body.

4-Methyl-2-pentanol or methyl isobutyl carbinol (MIBC) is an organic chemical compound used primarily as a frother in mineral flotation and in the production of lubricant oil additives such as Zinc dithiophosphate. It is also used as a solvent, in organic synthesis, and in the manufacture of brake fluid and as a precursor to some plasticizers. It is an acetone derivative in liquid state, with limited solubility in water but generally miscible with most organic solvents.
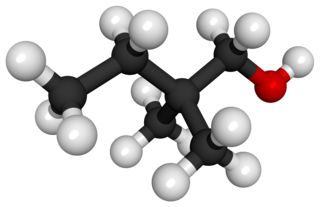
2,2-Dimethyl-1-butanol is an organic chemical compound; it is one of the isomeric hexanols. Its main use is as a solvent.

Sodium ferrioxalate is a chemical compound with the formula Na3Fe(C2O4)3. It often occurs as a hydrate such as Na3[Fe(C2O4)3]·nH2O, are lime green in colour. It is also called sodium oxalatoferrate or sodium trisoxalatoferrate.
3-Methyl-2-butanol is an organic chemical compound. It is used as a solvent and an intermediate in the manufacture of other chemicals.

Chromium(III) bromide is an inorganic compound with the chemical formula CrBr3. It is a dark colored solid that appears green in transmitted light but red with reflected light. It is used as a precursor to catalysts for the oligomerization of ethylene.