
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or in the terminal position. Terminal alkenes are also known as α-olefins.

Petrochemicals are the chemical products obtained from petroleum by refining. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as maize, palm fruit or sugar cane.
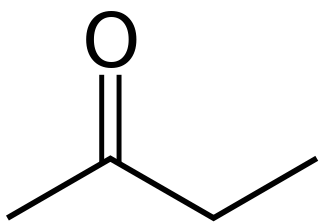
Butanone, also known as methyl ethyl ketone (MEK) or ethyl methyl ketone, is an organic compound with the formula CH3C(O)CH2CH3. This colorless liquid ketone has a sharp, sweet odor reminiscent of acetone. It is produced industrially on a large scale, but occurs in nature only in trace amounts. It is partially soluble in water, and is commonly used as an industrial solvent. It is an isomer of another solvent, tetrahydrofuran.

1,3-Butadiene is the organic compound with the formula CH2=CH-CH=CH2. It is a colorless gas that is easily condensed to a liquid. It is important industrially as a precursor to synthetic rubber. The molecule can be viewed as the union of two vinyl groups. It is the simplest conjugated diene.

Alkylation is a chemical reaction that entails transfer of an alkyl group. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene. Alkylating agents are reagents for effecting alkylation. Alkyl groups can also be removed in a process known as dealkylation. Alkylating agents are often classified according to their nucleophilic or electrophilic character. In oil refining contexts, alkylation refers to a particular alkylation of isobutane with olefins. For upgrading of petroleum, alkylation produces a premium blending stock for gasoline. In medicine, alkylation of DNA is used in chemotherapy to damage the DNA of cancer cells. Alkylation is accomplished with the class of drugs called alkylating antineoplastic agents.
Butene, also known as butylene, is an alkene with the formula C4H8. The word butene may refer to any of the individual compounds. They are colourless gases that are present in crude oil as a minor constituent in quantities that are too small for viable extraction. Butene is therefore obtained by catalytic cracking of long-chain hydrocarbons left during refining of crude oil. Cracking produces a mixture of products, and the butene is extracted from this by fractional distillation.
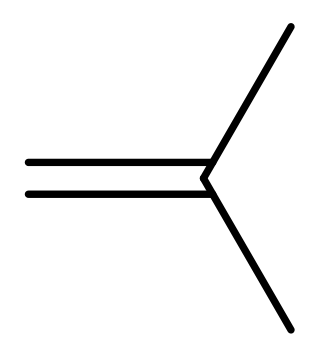
Isobutylene is a hydrocarbon with the chemical formula (CH3)2C=CH2. It is a four-carbon branched alkene (olefin), one of the four isomers of butylene. It is a colorless flammable gas, and is of considerable industrial value.
The Robinson annulation is a chemical reaction used in organic chemistry for ring formation. It was discovered by Robert Robinson in 1935 as a method to create a six membered ring by forming three new carbon–carbon bonds. The method uses a ketone and a methyl vinyl ketone to form an α,β-unsaturated ketone in a cyclohexane ring by a Michael addition followed by an aldol condensation. This procedure is one of the key methods to form fused ring systems.
An allylic rearrangement or allylic shift is an organic chemical reaction in which reaction at a center vicinal to a double bond causes the double bond to shift to an adjacent pair of atoms:
The Paternò–Büchi reaction, named after Emanuele Paternò and George Büchi, who established its basic utility and form, is a photochemical reaction, specifically a 2+2 photocycloaddition, which forms four-membered oxetane rings from an excited carbonyl and reacting with an alkene.

C5H10 is the molecular formula of 13 hydrocarbon isomers (represented by their CAS numbers on the chart). They can be divided into cycloalkanes and alkenes.
The molecular formula C6H12 may refer to following structural isomers:

Pinacolone (3,3-dimethyl-2-butanone) is an important ketone in organic chemistry. It is a colorless liquid and has a slight peppermint- or camphor- odor. It is a precursor to triazolylpinacolone in the synthesis of the fungicide triadimefon and in synthesis of the herbicide metribuzin. The molecule is an unsymmetrical ketone. The α-methyl group can participate in condensation reactions. The carbonyl group can undergo the usual reactions. It is a Schedule 3 compound under the Chemical Weapons Convention 1993, due to being related to pinacolyl alcohol, which is used in the production of soman. It is also a controlled export in Australia Group member states.
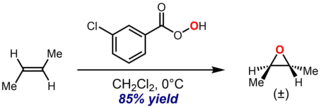
The Prilezhaev reaction, also known as the Prileschajew reaction or Prilezhaev epoxidation, is the chemical reaction of an alkene with a peroxy acid to form epoxides. It is named after Nikolai Prilezhaev, who first reported this reaction in 1909. A widely used peroxy acid for this reaction is meta-chloroperoxybenzoic acid (m-CPBA), due to its stability and good solubility in most organic solvents. The reaction is performed in inert solvents (C6H14, C6H6, CH2Cl2, CHCl3, CCl4) between -10 and 60 °C with the yield of 60-80%.

tert-Amyl alcohol (TAA) or 2-methylbutan-2-ol (2M2B), is a branched pentanol.
Lindgren oxidation is a selective method for oxidizing aldehydes to carboxylic acids. The reaction is named after Bengt O. Lindgren.
tert-Amyl methyl ether (TAME) is an ether used as a fuel oxygenate. TAME derives from C5 distillation fractions of naphtha. It has an ethereous odor. Unlike most ethers, it does not require a stabilizer as it does not form peroxides on storage.

1,2-Dimethylcyclopropane is a cycloalkane consisting of a cyclopropane ring substituted with two methyl groups attached to adjacent carbon atoms. It has three stereoisomers, one cis-isomer and a pair of trans-enantiomers, which differ depending on the orientation of the two methyl groups. As with other cyclopropanes, ring tension results in a relatively unstable compound.
George Hermann Büchi was a Swiss organic chemist and professor at the Massachusetts Institute of Technology. "Paternò's reaction", known since the early twentieth century, was renamed to the "Paternò–Büchi reaction" based on enhancements made to it by Büchi's research group.
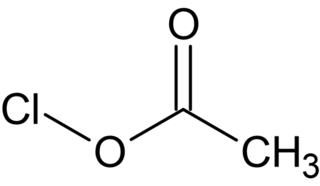
Acetyl hypochlorite, also known as chlorine acetate, is a chemical compound with the formula CH3COOCl. It is a photosensitive colorless liquid that is a short lived intermediate in the Hunsdiecker reaction.













