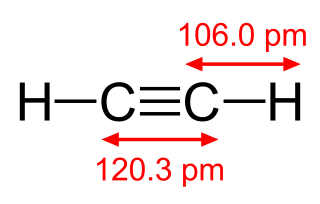
Acetylene is the chemical compound with the formula C2H2 and structure H−C≡C−H. It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pure form and thus is usually handled as a solution. Pure acetylene is odorless, but commercial grades usually have a marked odor due to impurities such as divinyl sulfide and phosphine.

Acetophenone is the organic compound with the formula C6H5C(O)CH3. It is the simplest aromatic ketone. This colorless, viscous liquid is a precursor to useful resins and fragrances.

Terpenes are a class of natural products consisting of compounds with the formula (C5H8)n for n ≥ 2. Terpenes are major biosynthetic building blocks. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predominantly by plants, particularly conifers. In plants, terpenes and terpenoids are important mediators of ecological interactions, while some insects use some terpenes as a form of defense. Other functions of terpenoids include cell growth modulation and plant elongation, light harvesting and photoprotection, and membrane permeability and fluidity control.

Alkylation is a chemical reaction that entails transfer of an alkyl group. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene. Alkylating agents are reagents for effecting alkylation. Alkyl groups can also be removed in a process known as dealkylation. Alkylating agents are often classified according to their nucleophilic or electrophilic character. In oil refining contexts, alkylation refers to a particular alkylation of isobutane with olefins. For upgrading of petroleum, alkylation produces a premium blending stock for gasoline. In medicine, alkylation of DNA is used in chemotherapy to damage the DNA of cancer cells. Alkylation is accomplished with the class of drugs called alkylating antineoplastic agents.

Propyne (methylacetylene) is an alkyne with the chemical formula CH3C≡CH. It is a component of MAPD gas—along with its isomer propadiene (allene), which was commonly used in gas welding. Unlike acetylene, propyne can be safely condensed.
In organometallic chemistry, acetylide refers to chemical compounds with the chemical formulas MC≡CH and MC≡CM, where M is a metal. The term is used loosely and can refer to substituted acetylides having the general structure RC≡CM. Acetylides are reagents in organic synthesis. The calcium acetylide commonly called calcium carbide is a major compound of commerce.

Acetone is an organic compound with the formula (CH3)2CO. It is the simplest and smallest ketone. It is a colorless, highly volatile and flammable liquid with a characteristic pungent odor.
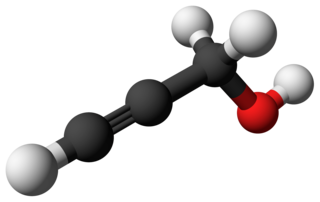
Propargyl alcohol, or 2-propyn-1-ol, is an organic compound with the formula C3H4O. It is the simplest stable alcohol containing an alkyne functional group. Propargyl alcohol is a colorless viscous liquid that is miscible with water and most polar organic solvents.

Organoaluminium chemistry is the study of compounds containing bonds between carbon and aluminium. It is one of the major themes within organometallic chemistry. Illustrative organoaluminium compounds are the dimer trimethylaluminium, the monomer triisobutylaluminium, and the titanium-aluminium compound called Tebbe's reagent. The behavior of organoaluminium compounds can be understood in terms of the polarity of the C−Al bond and the high Lewis acidity of the three-coordinated species. Industrially, these compounds are mainly used for the production of polyolefins.
The Favorskii reaction is an organic chemistry reaction between an alkyne and a carbonyl group, under basic conditions. The reaction was discovered in the early 1900s by the Russian chemist Alexei Yevgrafovich Favorskii.
In chemistry, carbonylation refers to reactions that introduce carbon monoxide (CO) into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemistry. The term carbonylation also refers to oxidation of protein side chains.

Tripotassium phosphate, also called tribasic potassium phosphate is a water-soluble salt with the chemical formula K3PO4.(H2O)x (x = 0, 3, 7, 9). Tripotassium phosphate is basic.

tert-Amyl alcohol (TAA) or 2-methylbutan-2-ol (2M2B), is a branched pentanol.

Trimethylsilylacetylene is the organosilicon compound with the formula (CH3)3SiC2H. A colorless liquid, "tms acetylene", as it is also called, is used as a source of "HC2−" in organic synthesis.
Alcohol oxidation is a collection of oxidation reactions in organic chemistry that convert alcohols to aldehydes, ketones, carboxylic acids, and esters where the carbon carries a higher oxidation state. The reaction mainly applies to primary and secondary alcohols. Secondary alcohols form ketones, while primary alcohols form aldehydes or carboxylic acids.

The Jones oxidation is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic acids and ketones, respectively. It is named after its discoverer, Sir Ewart Jones. The reaction was an early method for the oxidation of alcohols. Its use has subsided because milder, more selective reagents have been developed, e.g. Collins reagent.
In organic chemistry, Nef synthesis is the addition of sodium acetylides to aldehydes and ketones to yield propargyl alcohols. It is named for John Ulric Nef, who discovered the reaction in 1899.
In organic chemistry, alkynylation is an addition reaction in which a terminal alkyne is added to a carbonyl group to form an α-alkynyl alcohol.
Propynyllithium is an organolithium compound with the chemical formula LiC
2CH
3. It is a white solid that is soluble in 1,2-dimethoxyethane, and tetrahydrofuran. To preclude its degradation by oxygen and water, propynyllithium and its solutions are handled under inert gas. Although commonly depicted as a monomer, propynyllithium adopts a more complicated cluster structure as seen for many other organolithium compounds.
Hydroxymethylation is a chemical reaction that installs the CH2OH group. The transformation can be implemented in many ways and applies to both industrial and biochemical processes.

















