
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic.

An ester is a chemical compound derived from an acid in which at least one –OH hydroxyl group is replaced by an –O– alkyl (alkoxy) group, as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils.

In chemistry, a ketone is a functional group with the structure R2C=O, where R can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond). The simplest ketone is acetone (R = R' = methyl), with the formula CH3C(O)CH3. Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids (e.g., testosterone), and the solvent acetone.

An aldol condensation is a condensation reaction in organic chemistry in which an enol or an enolate ion reacts with a carbonyl compound to form a β-hydroxyaldehyde or β-hydroxyketone, followed by dehydration to give a conjugated enone.

An allyl group is a substituent with the structural formula H2C=CH−CH2R, where R is the rest of the molecule. It consists of a methylene bridge (−CH2−) attached to a vinyl group (−CH=CH2). The name is derived from the Latin word for garlic, Allium sativum. In 1844, Theodor Wertheim isolated an allyl derivative from garlic oil and named it "Schwefelallyl". The term allyl applies to many compounds related to H2C=CH−CH2, some of which are of practical or of everyday importance, for example, allyl chloride.
The Wolff–Kishner reduction is a reaction used in organic chemistry to convert carbonyl functionalities into methylene groups. In the context of complex molecule synthesis, it is most frequently employed to remove a carbonyl group after it has served its synthetic purpose of activating an intermediate in a preceding step. As such, there is no obvious retron for this reaction. The reaction was reported by Nikolai Kischner in 1911 and Ludwig Wolff in 1912,
Acetylide refers to chemical compounds with the chemical formulas MC≡CH and MC≡CM, where M is a metal. The term is used loosely and can refer to substituted acetylides having the general structure RC≡CM. Acetylides are reagents in organic synthesis. The calcium acetylide commonly called calcium carbide is a major compound of commerce.
Ozonolysis is an organic reaction where the unsaturated bonds of alkenes, alkynes, or azo compounds are cleaved with ozone. Alkenes and alkynes form organic compounds in which the multiple carbon–carbon bond has been replaced by a carbonyl group while azo compounds form nitrosamines. The outcome of the reaction depends on the type of multiple bond being oxidized and the work-up conditions.
The Cannizzaro reaction, named after its discoverer Stanislao Cannizzaro, is a chemical reaction which involves the base-induced disproportionation of two molecules of a non-enolizable aldehyde to give a primary alcohol and a carboxylic acid.
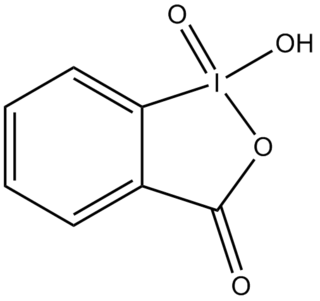
2-Iodoxybenzoic acid (IBX) is an organic compound used in organic synthesis as an oxidizing agent. This periodinane is especially suited to oxidize alcohols to aldehydes. IBX is prepared from 2-iodobenzoic acid, potassium bromate, and sulfuric acid. Frigerio and co-workers have also demonstrated, in 1999 that potassium bromate may be replaced by commercially available Oxone. One of the main drawbacks of IBX is its limited solubility; IBX is insoluble in many common organic solvents. In the past, it was believed that IBX was shock sensitive, but it was later proposed that samples of IBX were shock sensitive due to the residual potassium bromate left from its preparation. Commercial IBX is stabilized by carboxylic acids such as benzoic acid and isophthalic acid.
The Favorskii rearrangement is principally a rearrangement of cyclopropanones and α-halo ketones that leads to carboxylic acid derivatives. In the case of cyclic α-halo ketones, the Favorskii rearrangement constitutes a ring contraction. This rearrangement takes place in the presence of a base, sometimes hydroxide, to yield a carboxylic acid but most of the time either an alkoxide base or an amine to yield an ester or an amide, respectively. α,α'-Dihaloketones eliminate HX under the reaction conditions to give α,β-unsaturated carbonyl compounds.

The Danishefsky Taxol total synthesis in organic chemistry is an important third Taxol synthesis published by the group of Samuel Danishefsky in 1996 two years after the first two efforts described in the Holton Taxol total synthesis and the Nicolaou Taxol total synthesis. Combined they provide a good insight in the application of organic chemistry in total synthesis.
The Meyer–Schuster rearrangement is the chemical reaction described as an acid-catalyzed rearrangement of secondary and tertiary propargyl alcohols to α,β-unsaturated ketones if the alkyne group is internal and α,β-unsaturated aldehydes if the alkyne group is terminal. Reviews have been published by Swaminathan and Narayan, Vartanyan and Banbanyan, and Engel and Dudley, the last of which describes ways to promote the Meyer–Schuster rearrangement over other reactions available to propargyl alcohols.
A homologation reaction, also known as homologization, is any chemical reaction that converts the reactant into the next member of the homologous series. A homologous series is a group of compounds that differ by a constant unit, generally a (-CH2-) group. The reactants undergo a homologation when the number of a repeated structural unit in the molecules is increased. The most common homologation reactions increase the number of methylene (-CH2-) units in saturated chain within the molecule. For example, the reaction of aldehydes or ketones with diazomethane or methoxymethylenetriphenylphosphine to give the next homologue in the series.
Luche reduction is the selective organic reduction of α,β-unsaturated ketones to allylic alcohols with sodium borohydride (NaBH4) and lanthanide chlorides, mainly cerium(III) chloride (CeCl3), in methanol or ethanol. The Luche reduction can be conducted chemoselectively toward ketone in the presence of aldehyde or toward α,β-unsaturated ketone in the presence of non-conjugated ketone.

In organic chemistry, carbonyl reduction is the organic reduction of any carbonyl group by a reducing agent.

The Jones oxidation is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic acids and ketones, respectively. It is named after its discoverer, Sir Ewart Jones. The reaction was an early method for the oxidation of alcohols. Its use has subsided because milder, more selective reagents have been developed, e.g. Collins reagent.
The asymmetric addition of alkynylzinc compounds to aldehydes is an example of a Nef synthesis, a chemical reaction whereby a chiral propargyl alcohol is prepared from a terminal alkyne and an aldehyde. This alkynylation reaction is enantioselective and involves an alkynylzinc reagent rather than the sodium acetylide used by John Ulric Nef in his 1899 report of the synthetic approach. Propargyl alcohols are versatile precursors for the chirally-selective synthesis of natural products and pharmaceutical agents, making this asymmetric addition reaction of alkynylzinc compounds useful. For example, Erick Carreira used this approach in a total synthesis of the marine natural product leucascandrolide A, a bioactive metabolite of the calcareous sponge Leucascandra caveolata with cytotoxic and antifungal properties isolated in 1996.
Alkynylation is an addition reaction in organic synthesis where a terminal alkyne adds to a carbonyl group to form an α-alkynyl alcohol. When the acetylide is formed from acetylene, the reaction gives an α-ethynyl alcohol. This process is often referred to as ethynylation. Such process often involve metal acetylide intermediates
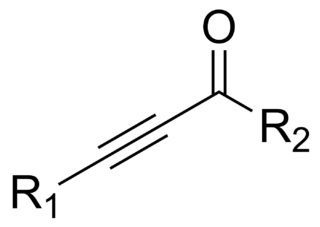
In organic chemistry, an ynone is a compound containing a ketone function and a C≡C triple bond. Many ynones are α,β-ynones, where the carbonyl and alkyne groups are conjugated. Capillin is a naturally occurring example. Some ynones are not conjugated.










