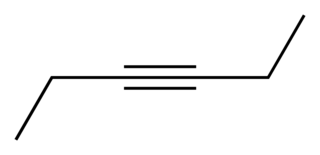In organic chemistry, hydrocarbons are divided into two classes: aromatic compounds and aliphatic compounds, also known as non-aromatic hydrocarbons. Aliphatics can be cyclic; however, hydrocarbons with conjugated pi-systems that obey Hückel's rule are instead considered to demonstrate aromaticity. Aliphatic compounds can be saturated, like hexane, or unsaturated, like hexene and hexyne. Open-chain compounds contain no rings of any type, and are thus aliphatic.

In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula CnH2n−2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic.

In stereochemistry, stereoisomerism, or spatial isomerism, is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms in space. This contrasts with structural isomers, which share the same molecular formula, but the bond connections or their order differs. By definition, molecules that are stereoisomers of each other represent the same structural isomer.
In chemical nomenclature, the IUPAC nomenclature of organic chemistry is a method of naming organic chemical compounds as recommended by the International Union of Pure and Applied Chemistry (IUPAC). It is published in the Nomenclature of Organic Chemistry. Ideally, every possible organic compound should have a name from which an unambiguous structural formula can be created. There is also an IUPAC nomenclature of inorganic chemistry.
The molecular formula C6H6 (molar mass: 78.114)
Hexene is an alkene with a molecular formula C6H12. The prefix "hex" is derived from the fact that there are 6 carbon atoms in the molecule, while the "-ene" suffix denotes that there is an alkene present—two carbon atoms are connected via a double bond. There are several isomers of hexene, depending on the position and geometry of the double bond in the chain. One of the most common industrially useful isomers is 1-hexene, an alpha-olefin. Hexene is used as a comonomer in the production of polyethylene.

1-Butyne, also known as ethylacetylene, but-1-yne, ethylethyne, and UN 2452, is an extremely flammable and reactive alkyne with chemical formula C4H6 and CAS number 107-00-6 that is used in the synthesis of organic compounds. It occurs as a colorless gas.

2-Butyne (dimethylacetylene, crotonylene or but-2-yne) is an alkyne with chemical formula CH3C≡CCH3. Produced artificially, it is a colorless, volatile, pungent liquid at standard temperature and pressure.

In chemistry, the suffix -yne is used to denote the presence of a triple bond.
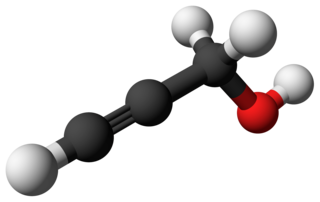
Propargyl alcohol, or 2-propyn-1-ol, is an organic compound with the formula C3H4O. It is the simplest stable alcohol containing an alkyne functional group. Propargyl alcohol is a colorless viscous liquid that is miscible with water and most polar organic solvents.
The molecular formula C6H10 (molar mass: 82.14 g/mol) may refer to:
In enzymology, an isohexenylglutaconyl-CoA hydratase (EC 4.2.1.57) is an enzyme that catalyzes the chemical reaction

Ethylmagnesium bromide is a Grignard reagent with formula C2H5MgBr. It is widely used in the laboratory synthesis of organic compounds.
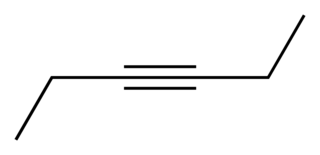
3-Hexyne is the organic compound with the formula C2H5CCC2H5. This colorless liquid is one of three isomeric hexynes. 3-Hexyne forms with 5-decyne, 4-octyne, and 2-butyne a series of symmetric alkynes. It is a reagent in organometallic chemistry.
In organic chemistry, the term ethynyl designates

3-Hexanol is an organic chemical compound. It occurs naturally in the flavor and aroma of plants such as pineapple and is used as a food additive to add flavor.

The thiol-yne reaction is an organic reaction between a thiol and an alkyne. The reaction product is an alkenyl sulfide. The reaction was first reported in 1949 with thioacetic acid as reagent and rediscovered in 2009. It is used in click chemistry and in polymerization, especially with dendrimers.

Four-carbon molecules are based on a skeleton made from four carbon atoms. They may be in a chain, branched chains, cycles or even bicyclic compounds
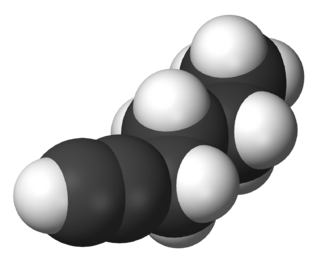
1-Hexyne (n-butylacetylene) is a hydrocarbon consisting of a straight six-carbon chain having a terminal alkyne. Its molecular formula is C6H10. It is a liquid at room temperature that is colorless or pale yellow in appearance.
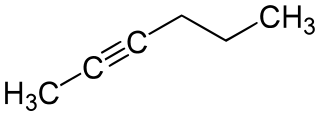
2-Hexyne is an organic compound that belongs to the alkyne group. Just like its isomers, it also has the chemical formula of C6H10.
This page is based on this
Wikipedia article Text is available under the
CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.