
In chemistry, an alkene is a hydrocarbon that contains a carbon–carbon double bond.
A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and plus (+) and minus (−) signs. These are limited to a single typographic line of symbols, which may include subscripts and superscripts. A chemical formula is not a chemical name, and it contains no words. Although a chemical formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. Chemical formulae can fully specify the structure of only the simplest of molecules and chemical substances, and are generally more limited in power than chemical names and structural formulae.
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic chemical compound. It is a simple alcohol with the chemical formula C2H6O. Its formula can be also written as CH
3−CH
2−OH or C
2H
5OH (an ethyl group linked to a hydroxyl group), and is often abbreviated as EtOH. Ethanol is a volatile, flammable, colorless liquid with a slight characteristic odor. It is a psychoactive substance, recreational drug, and the active ingredient in alcoholic drinks.
In chemistry, the empirical formula of a chemical compound is the simplest positive integer ratio of atoms present in a compound. A simple example of this concept is that the empirical formula of sulfur monoxide, or SO, would simply be SO, as is the empirical formula of disulfur dioxide, S2O2. Thus, sulfur monoxide and disulfur dioxide, both compounds of sulphur and oxygen, have the same empirical formula. However, their molecular formulas, which express the number of atoms in each molecule of a chemical compound, are not the same.

In chemistry, a ketone is a functional group with the structure R2C=O, where R can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond). The simplest ketone is acetone (R = R' = methyl), with the formula CH3C(O)CH3. Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids (e.g., testosterone), and the solvent acetone.
A methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms — CH3. In formulas, the group is often abbreviated Me. Such hydrocarbon groups occur in many organic compounds. It is a very stable group in most molecules. While the methyl group is usually part of a larger molecule, it can be found on its own in any of three forms: anion, cation or radical. The anion has eight valence electrons, the radical seven and the cation six. All three forms are highly reactive and rarely observed.
Chemically, an aldehyde is a compound containing a functional group with the structure −CHO, consisting of a carbonyl center with the carbon atom also bonded to hydrogen and to any generic alkyl or side chain R group,. The functional group itself is known as an aldehyde or formyl group.
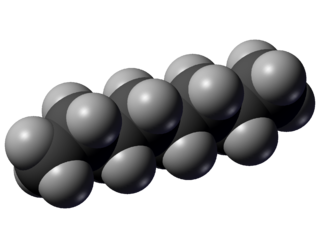
Octane is a hydrocarbon and an alkane with the chemical formula C8H18, and the condensed structural formula CH3(CH2)6CH3. Octane has many structural isomers that differ by the amount and location of branching in the carbon chain. One of these isomers, 2,2,4-trimethylpentane (commonly called iso-octane) is used as one of the standard values in the octane rating scale.
Unsymmetrical dimethylhydrazine (UDMH; 1,1-dimethylhydrazine ; heptyl) is a chemical compound with the formula H2NN(CH3)2 that is used as a rocket propellant. It is a colorless liquid, with a sharp, fishy, ammonia-like smell typical for organic amines. Samples turn yellowish on exposure to air and absorb oxygen and carbon dioxide. It is miscible with water, ethanol, and kerosene. In concentration between 2.5% and 95% in air, its vapors are flammable. It is not sensitive to shock. Symmetrical dimethylhydrazine, 1,2-dimethylhydrazine is also known but is not as useful.
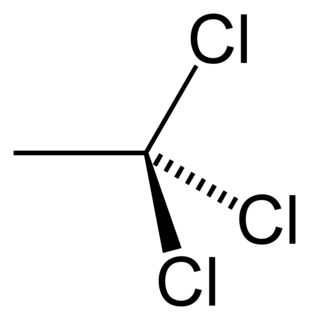
The organic compound 1,1,1-trichloroethane, also known as methyl chloroform, is a chloroalkane. This colourless, sweet-smelling liquid was once produced industrially in large quantities for use as a solvent. It is regulated by the Montreal Protocol as an ozone-depleting substance and its use is being rapidly phased out.

Ethylene oxide is an organic compound with the formula C
2H
4O. It is a cyclic ether and the simplest epoxide: a three-membered ring consisting of one oxygen atom and two carbon atoms. Ethylene oxide is a colorless and flammable gas with a faintly sweet odor. Because it is a strained ring, ethylene oxide easily participates in a number of addition reactions that result in ring-opening. Ethylene oxide is isomeric with acetaldehyde and with vinyl alcohol. Ethylene oxide is industrially produced by oxidation of ethylene in the presence of silver catalyst.

Dimethylmercury ((CH3)2Hg) is an extremely toxic organomercury compound. A highly volatile, reactive, flammable, and colorless liquid, dimethylmercury is one of the strongest known neurotoxins, with a quantity of less than 0.1 mL capable of inducing severe mercury poisoning resulting in death, and is easily absorbed through the skin. Dimethylmercury is capable of permeating many materials, including plastic and rubber compounds. It has a slightly sweet odor, although inhaling enough of the chemical to notice this would be hazardous.
In chemistry, halogenation is a chemical reaction that involves the addition of one or more halogens to a compound or material. The pathway and stoichiometry of halogenation depends on the structural features and functional groups of the organic substrate, as well as on the specific halogen. Inorganic compounds such as metals also undergo halogenation.
The chemical compound 1,2-dichloroethane, commonly known as ethylene dichloride (EDC), is a chlorinated hydrocarbon. It is a colourless liquid with a chloroform-like odour. The most common use of 1,2-dichloroethane is in the production of vinyl chloride, which is used to make polyvinyl chloride (PVC) pipes, furniture and automobile upholstery, wall coverings, housewares, and automobile parts. 1,2-Dichloroethane is also used generally as an intermediate for other organic chemical compounds, and as a solvent. It forms azeotropes with many other solvents, including water and other chlorocarbons.

2-Methylheptane is a branched alkane isomeric to octane. Its structural formula is (CH3)2CH(CH2)4CH3.
Organoarsenic chemistry is the chemistry of compounds containing a chemical bond between arsenic and carbon. A few organoarsenic compounds, also called "organoarsenicals," are produced industrially with uses as insecticides, herbicides, and fungicides. In general these applications are declining in step with growing concerns about their impact on the environment and human health. The parent compounds are arsine and arsenic acid. Despite their toxicity, organoarsenic biomolecules are well known.

In chemistry, isomers are molecules or polyatomic ions with identical molecular formulas — that is, same number of atoms of each element — but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers.
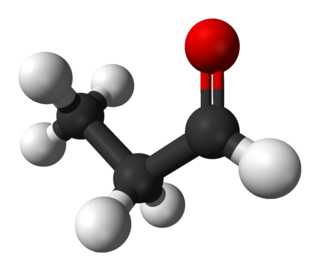
Propionaldehyde or propanal is the organic compound with the formula CH3CH2CHO. It is the 3-carbon aldehyde. It is a colourless, flammable liquid with a slightly fruity odour. It is produced on a large scale industrially.
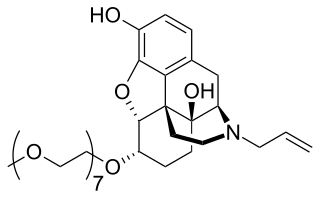
Naloxegol is a peripherally acting μ-opioid receptor antagonist developed by AstraZeneca, licensed from Nektar Therapeutics, for the treatment of opioid-induced constipation. It was approved in 2014 in adult patients with chronic, non-cancer pain. Doses of 25 mg were found safe and well tolerated for 52 weeks. When given concomitantly with opioid analgesics, naloxegol reduced constipation-related side effects, while maintaining comparable levels of analgesia.
Propionitrile, also known as ethyl cyanide and propanenitrile, is an organic compound with the formula CH3CH2CN. It is a simple aliphatic nitrile. The compound is a colourless, water-soluble liquid. It is used as a solvent and a precursor to other organic compounds.















