
Selective laser sintering (SLS) is an additive manufacturing (AM) technique that uses a laser as the power and heat source to sinter powdered material, aiming the laser automatically at points in space defined by a 3D model, binding the material together to create a solid structure. It is similar to selective laser melting; the two are instantiations of the same concept but differ in technical details. SLS is a relatively new technology that so far has mainly been used for rapid prototyping and for low-volume production of component parts. Production roles are expanding as the commercialization of AM technology improves.

3D printing or additive manufacturing is the construction of a three-dimensional object from a CAD model or a digital 3D model. It can be done in a variety of processes in which material is deposited, joined or solidified under computer control, with the material being added together, typically layer by layer.

Organ printing utilizes techniques similar to conventional 3D printing where a computer model is fed into a printer that lays down successive layers of plastics or wax until a 3D object is produced. In the case of organ printing, the material being used by the printer is a biocompatible plastic. The biocompatible plastic forms a scaffold that acts as the skeleton for the organ that is being printed. As the plastic is being laid down, it is also seeded with human cells from the patient's organ that is being printed for. After printing, the organ is transferred to an incubation chamber to give the cells time to grow. After a sufficient amount of time, the organ is implanted into the patient.
Electron-beam additive manufacturing, or electron-beam melting (EBM) is a type of additive manufacturing, or 3D printing, for metal parts. The raw material is placed under a vacuum and fused together from heating by an electron beam. This technique is distinct from selective laser sintering as the raw material fuses having completely melted.

Stratasys, Ltd. is an American-Israeli manufacturer of 3D printers, software, and materials for polymer additive manufacturing as well as 3D-printed parts on-demand. The company is incorporated in Israel. Engineers use Stratasys systems to model complex geometries in a wide range of polymer materials, including: ABS, polyphenylsulfone (PPSF), polycarbonate (PC) and polyetherimide and Nylon 12.

Pharmaceutical manufacturing is the process of industrial-scale synthesis of pharmaceutical drugs as part of the pharmaceutical industry. The process of drug manufacturing can be broken down into a series of unit operations, such as milling, granulation, coating, tablet pressing, and others.

Binder jet 3D printing, known variously as "Powder bed and inkjet" and "drop-on-powder" printing, is a rapid prototyping and additive manufacturing technology for making objects described by digital data such as a CAD file. Binder jetting is one of the seven categories of additive manufacturing processes according to ASTM and ISO.
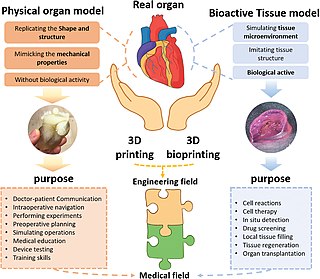
Three dimensional (3D) bioprinting is the utilization of 3D printing–like techniques to combine cells, growth factors, bio-inks, and/or biomaterials to fabricate biomedical parts that imitate natural tissue characteristics, form functional biofilms, and assist in the removal of pollutants. 3D bioprinting has uses in fields such as wastewater treatment, environmental remediation, and corrosion prevention. 3D bioprinting can produce functional biofilms which can assist in a variety of situations. The 3D bioprinted biofilms host functional microorganisms which can facilitate pollutant removal. Generally, 3D bioprinting can utilize a layer-by-layer method to deposit materials known as bio-inks to create tissue-like structures that are later used in various medical and tissue engineering fields. 3D bioprinting covers a broad range of bioprinting techniques and biomaterials. Currently, bioprinting can be used to print tissue and organ models to help research drugs and potential treatments. Nonetheless, translation of bioprinted living cellular constructs into clinical application is met with several issues due to the complexity and cell number necessary to create functional organs. However, innovations span from bioprinting of extracellular matrix to mixing cells with hydrogels deposited layer by layer to produce the desired tissue. In addition, 3D bioprinting has begun to incorporate the printing of scaffolds which can be used to regenerate joints and ligaments.
Robocasting is an additive manufacturing technique analogous to Direct Ink Writing and other extrusion-based 3D-printing techniques in which a filament of a paste-like material is extruded from a small nozzle while the nozzle is moved across a platform. The object is thus built by printing the required shape layer by layer. The technique was first developed in the United States in 1996 as a method to allow geometrically complex ceramic green bodies to be produced by additive manufacturing. In robocasting, a 3D CAD model is divided up into layers in a similar manner to other additive manufacturing techniques. The material is then extruded through a small nozzle as the nozzle's position is controlled, drawing out the shape of each layer of the CAD model. The material exits the nozzle in a liquid-like state but retains its shape immediately, exploiting the rheological property of shear thinning. It is distinct from fused deposition modelling as it does not rely on the solidification or drying to retain its shape after extrusion.
Fused filament fabrication (FFF), also known as fused deposition modeling, or filament freeform fabrication, is a 3D printing process that uses a continuous filament of a thermoplastic material. Filament is fed from a large spool through a moving, heated printer extruder head, and is deposited on the growing work. The print head is moved under computer control to define the printed shape. Usually the head moves in two dimensions to deposit one horizontal plane, or layer, at a time; the work or the print head is then moved vertically by a small amount to begin a new layer. The speed of the extruder head may also be controlled to stop and start deposition and form an interrupted plane without stringing or dribbling between sections. "Fused filament fabrication" was coined by the members of the RepRap project to give an acronym (FFF) that would be legally unconstrained in its use.

In recent years, 3D printing has developed significantly and can now perform crucial roles in many applications, with the most common applications being manufacturing, medicine, architecture, custom art and design, and can vary from fully functional to purely aesthetic applications.

A variety of processes, equipment, and materials are used in the production of a three-dimensional object via additive manufacturing. 3D printing is also known as additive manufacturing, because the numerous available 3D printing process tend to be additive in nature, with a few key differences in the technologies and the materials used in this process.
Material extrusion-based additive manufacturing (EAM) represents one of the seven categories of 3d printing processes, defined by the ISO international standard 17296-2. While it is mostly used for plastics, under the name of FDM or FFF, it can also be used for metals and ceramics. In this AM process category, the feedstock materials are mixtures of a polymeric binder and a fine grain solid powder of metal or ceramic materials. Similar type of feedstock is also used in the Metal Injection Molding (MIM) and in the Ceramic Injection Molding (CIM) processes. The extruder pushes the material towards a heated nozzle thanks to
3D printing speed measures the amount of manufactured material over a given time period, where the unit of time is measured in Seconds, and the unit of manufactured material is typically measured in units of either kg, mm or cm3, depending on the type of additive manufacturing technique.
Multi-material 3D printing is the additive manufacturing procedure of using multiple materials at the same time to fabricate an object. Similar to single material additive manufacturing it can be realised through methods such as FFF, SLA and Inkjet 3D printing. By expanding the design space to different materials, it establishes the possibilities of creating 3D printed objects of different color or with different material properties like elasticity or solubility. The first multi-material 3D printer Fab@Home became publicly available in 2006. The concept was quickly adopted by the industry followed by many consumer ready multi-material 3D printers.

3D food printing is the process of manufacturing food products using a variety of additive manufacturing techniques. Most commonly, food grade syringes hold the printing material, which is then deposited through a food grade nozzle layer by layer. The most advanced 3D food printers have pre-loaded recipes on board and also allow the user to remotely design their food on their computers, phones or some IoT device. The food can be customized in shape, color, texture, flavor or nutrition, which makes it very useful in various fields such as space exploration and healthcare.
Abdul Waseh Basit is a professor of pharmaceutics at University College London, and founder of two pharmaceutical biotechnology companies spinning out of UCL. Basit is interested in particular in oral drug delivery and pharmaceutical three-dimensional (3D) printing.

3D concrete printing, or simply concrete printing, refers to digital fabrication processes for cementitious materials based on one of several different 3D printing technologies. 3D printed concrete eliminates the need for formwork, reducing material waste and allowing for greater geometric freedom in complex structures. With recent developments in mix design and 3D printing technology over the last decade, 3D concrete printing has grown exponentially since its emergence in the 1990s. Architectural and structural applications of 3D-printed concrete include the production of building blocks, building modules, street furniture, pedestrian bridges, and low-rise residential structures.
Bioprinting drug delivery is a method of using the three-dimensional printing of biomaterials through an additive manufacturing technique to develop drug delivery vehicles that are biocompatible tissue-specific hydrogels or implantable devices. 3D bioprinting uses printed cells and biological molecules to manufacture tissues, organs, or biological materials in a scaffold-free manner that mimics living human tissue to provide localized and tissue-specific drug delivery, allowing for targeted disease treatments with scalable and complex geometry.
3D printed medicine refers to medicine manufactured using 3D printing technology. It includes 3D printed medications or 3D printed drugs, which are medications that are manufactured using 3D printing technology. 3D printing enables the creation of customized and precise dosage forms tailored to the specific needs of patients. Various technologies have been developed to create 3D-printed medications.










