Related Research Articles
In toxicology, the median lethal dose, LD50 (abbreviation for "lethal dose, 50%"), LC50 (lethal concentration, 50%) or LCt50 is a toxic unit that measures the lethal dose of a given substance. The value of LD50 for a substance is the dose required to kill half the members of a tested population after a specified test duration. LD50 figures are frequently used as a general indicator of a substance's acute toxicity. A lower LD50 is indicative of higher toxicity.

Pharmacology is a science of medical drug and medication, including a substance's origin, composition, pharmacokinetics, therapeutic use, and toxicology. More specifically, it is the study of the interactions that occur between a living organism and chemicals that affect normal or abnormal biochemical function. If substances have medicinal properties, they are considered pharmaceuticals.
The therapeutic index is a quantitative measurement of the relative safety of a drug. It is a comparison of the amount of a therapeutic agent that causes the therapeutic effect to the amount that causes toxicity. The related terms therapeutic window or safety window refer to a range of doses optimized between efficacy and toxicity, achieving the greatest therapeutic benefit without resulting in unacceptable side-effects or toxicity.
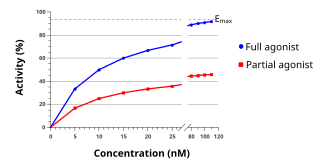
An agonist is a chemical that activates a receptor to produce a biological response. Receptors are cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an antagonist blocks the action of the agonist, while an inverse agonist causes an action opposite to that of the agonist.
In toxicology, the lethal dose (LD) is an indication of the lethal toxicity of a given substance or type of radiation. Because resistance varies from one individual to another, the "lethal dose" represents a dose at which a given percentage of subjects will die. The lethal concentration is a lethal dose measurement used for gases or particulates. The LD may be based on the standard person concept, a theoretical individual that has perfectly "normal" characteristics, and thus not apply to all sub-populations.

A receptor antagonist is a type of receptor ligand or drug that blocks or dampens a biological response by binding to and blocking a receptor rather than activating it like an agonist. Antagonist drugs interfere in the natural operation of receptor proteins. They are sometimes called blockers; examples include alpha blockers, beta blockers, and calcium channel blockers. In pharmacology, antagonists have affinity but no efficacy for their cognate receptors, and binding will disrupt the interaction and inhibit the function of an agonist or inverse agonist at receptors. Antagonists mediate their effects by binding to the active site or to the allosteric site on a receptor, or they may interact at unique binding sites not normally involved in the biological regulation of the receptor's activity. Antagonist activity may be reversible or irreversible depending on the longevity of the antagonist–receptor complex, which, in turn, depends on the nature of antagonist–receptor binding. The majority of drug antagonists achieve their potency by competing with endogenous ligands or substrates at structurally defined binding sites on receptors.
Pharmacodynamics (PD) is the study of the biochemical and physiologic effects of drugs. The effects can include those manifested within animals, microorganisms, or combinations of organisms.
Therapeutic drug monitoring (TDM) is a branch of clinical chemistry and clinical pharmacology that specializes in the measurement of medication levels in blood. Its main focus is on drugs with a narrow therapeutic range, i.e. drugs that can easily be under- or overdosed. TDM aimed at improving patient care by individually adjusting the dose of drugs for which clinical experience or clinical trials have shown it improved outcome in the general or special populations. It can be based on a a priori pharmacogenetic, demographic and clinical information, and/or on the a posteriori measurement of blood concentrations of drugs (pharmacokinetic monitoring) or biological surrogate or end-point markers of effect (pharmacodynamic monitoring).
Acute toxicity describes the adverse effects of a substance that result either from a single exposure or from multiple exposures in a short period of time. To be described as acute toxicity, the adverse effects should occur within 14 days of the administration of the substance.

Epibatidine is a chlorinated alkaloid that is secreted by the Ecuadoran frog Epipedobates anthonyi and poison dart frogs from the Ameerega genus. It was discovered by John W. Daly in 1974, but its structure was not fully elucidated until 1992. Whether epibatidine is the first observed example of a chlorinated alkaloid remains controversial, due to challenges in conclusively identifying the compound from the limited samples collected by Daly. By the time that high-resolution spectrometry was used in 1991, there remained less than one milligram of extract from Daly's samples, raising concerns about possible contamination. Samples from other batches of the same species of frog failed to yield epibatidine.
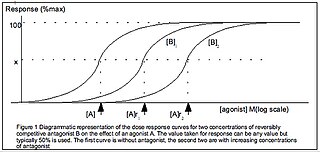
Half maximal effective concentration (EC50) is a measure of the concentration of a drug, antibody or toxicant which induces a biological response halfway between the baseline and maximum after a specified exposure time. More simply, EC50 can be defined as the concentration required to obtain a 50% [...] effect and may be also written as [A]50. It is commonly used as a measure of a drug's potency, although the use of EC50 is preferred over that of 'potency', which has been criticised for its vagueness. EC50 is a measure of concentration, expressed in molar units (M), where 1 M is equivalent to 1 mol/L.

The dose–response relationship, or exposure–response relationship, describes the magnitude of the response of an organism, as a function of exposure to a stimulus or stressor after a certain exposure time. Dose–response relationships can be described by dose–response curves. This is explained further in the following sections. A stimulus response function or stimulus response curve is defined more broadly as the response from any type of stimulus, not limited to chemicals.

In pharmacology, potency or biological potency is a measure of a drug's biological activity expressed in terms of the dose required to produce a pharmacological effect of given intensity. A highly potent drug evokes a given response at low concentrations, while a drug of lower potency evokes the same response only at higher concentrations. Higher potency does not necessarily mean greater effectiveness or more side effects.
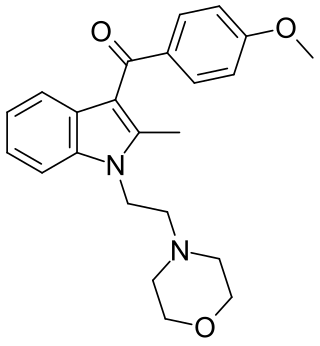
Pravadoline (WIN 48,098) is an anti-inflammatory and analgesic drug with an IC50 of 4.9 μM and a Ki of 2511 nM at CB1, related in structure to nonsteroidal anti-inflammatory drugs (NSAIDs) such as indometacin. It was developed in the 1980s as a new antiinflammatory and prostaglandin synthesis inhibitor, acting through inhibition of the enzyme cyclooxygenase (COX).
The protective index is a comparison of the amount of a therapeutic agent that causes the therapeutic effect to the amount that causes toxicity. Quantitatively, it is the ratio given by the toxic dose divided by the therapeutic dose. A protective index is the toxic dose of a drug for 50% of the population (TD50) divided by the minimum effective dose for 50% of the population (ED50). A high protective index is preferable to a low one: this corresponds to a situation in which one would have to take a much higher dose of a drug to reach the toxic threshold than the dose taken to elicit the therapeutic effect. A drug should ordinarily only be administered if the protective index is greater than one, indicating that the benefit outweighs the risk.

In toxicology, the median toxic dose (TD50) of a drug or toxin is the dose at which toxicity occurs in 50% of cases. The type of toxicity should be specified for this value to have meaning for practical purposes. The median toxic dose encompasses the category of toxicity that is greater than half maximum effective concentration (ED50) but less than the median lethal dose (LD50). However, for some highly potent toxins (ex. lofentanil, botulinum toxin) the difference between the ED50 and TD50 is so minute that the values assigned to them may be approximated to equal doses. Since toxicity need not be lethal, the TD50 is generally lower than the median lethal dose (LD50), and the latter can be considered an upper bound for the former. However, since the toxicity is above the effective limit, the TD50 is generally greater than the ED50. If the result of a study is a toxic effect that does not result in death, it is classified as this form of toxicity. Toxic effects can be defined differently, sometimes considering the therapeutic effect of a substance to be toxic (such as with chemotherapeutics) which can lead to confusion and contention regarding a substance's TD50. Examples of these toxic endpoints include cancer, blindness, anemia, and birth defects.

The phases of clinical research are the stages in which scientists conduct experiments with a health intervention to obtain sufficient evidence for a process considered effective as a medical treatment. For drug development, the clinical phases start with testing for drug safety in a few human subjects, then expand to many study participants to determine if the treatment is effective. Clinical research is conducted on drug candidates, vaccine candidates, new medical devices, and new diagnostic assays.

N,N-Dimethyldopamine (DMDA) is an organic compound belonging to the phenethylamine family. It is related structurally to the alkaloid epinine (N-methyldopamine) and to the major neurotransmitter dopamine (of which it is the N,N-dimethylated analog). Because of its structural relationship to dopamine, DMDA has been the subject of a number of pharmacological investigations. DMDA has been detected in Acacia rigidula.

Lomerizine (INN) is a diphenylpiperazine class L-type and T-type calcium channel blocker. This drug is currently used clinically for the treatment of migraines, while also being used experimentally for the treatment of glaucoma and optic nerve injury.
Threshold dose is the minimum dose of drug that triggers minimal detectable biological effect in an animal. At extremely low doses, biological responses are absent for some of the drugs. The increase in dose above threshold dose induces an increase in the percentage of biological responses. Several benchmarks have been established to describe the effects of a particular dose of drug in a particular species, such as NOEL(no-observed-effect-level), NOAEL(no-observed-adverse-effect-level) and LOAEL(lowest-observed-adverse-effect-level). They are established by reviewing the available studies and animal studies. The application of threshold dose in risk assessment safeguards the participants in human clinical trials and evaluates the risks of chronic exposure to certain substances. However, the nature of animal studies also limits the applicability of experimental results in the human population and its significance in evaluating potential risk of certain substances. In toxicology, there are some other safety factors including LD50, LC50 and EC50.
References
- ↑ Filloon, T. G. (May 1995). "Estimating the minimum therapeutically effective dose of a compound via regression modelling and percentile estimation". Statistics in Medicine. 14 (9–10): 925–932, discussion 933. doi:10.1002/sim.4780140911. ISSN 0277-6715. PMID 7569511.
- ↑ Street, Farnam (2014-02-13). "The Minimum Effective Dose: Why Less is More". Farnam Street. Retrieved 2023-05-23.
- ↑ Rang HP, Dale MM, Flower RJ, Henderson G (2015-01-21). Rang and Dale's pharmacology (Eighth ed.). United Kingdom: Elsevier Churchill Livingstone. ISBN 9780702053627. OCLC 903083639.
- ↑ "Woman Dies After Water-drinking Contest". NBC. Associated Press.
- 1 2 3 Liu J (2010). "Minimum Effective Dose". In Chow S (ed.). Encyclopedia of Biopharmaceutical Statistics. Taylor & Francis. doi:10.1081/E-EBS3. ISBN 978-1-4398-2246-3.
- ↑ IUPAC , Compendium of Chemical Terminology , 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) " median effective dose, ED50 ". doi : 10.1351/goldbook.M03808
- ↑ Miller R, Eriksson L, Fleisher L, Wiener-Kronish J, Young W (May 2009). Miller's Anesthesia (7th ed.). Churchill Livingstone. pp. 500–504. ISBN 978-1-4557-0876-5.