
Eicosanoids are signaling molecules made by the enzymatic or non-enzymatic oxidation of arachidonic acid or other polyunsaturated fatty acids (PUFAs) that are, similar to arachidonic acid, around 20 carbon units in length. Eicosanoids are a sub-category of oxylipins, i.e. oxidized fatty acids of diverse carbon units in length, and are distinguished from other oxylipins by their overwhelming importance as cell signaling molecules. Eicosanoids function in diverse physiological systems and pathological processes such as: mounting or inhibiting inflammation, allergy, fever and other immune responses; regulating the abortion of pregnancy and normal childbirth; contributing to the perception of pain; regulating cell growth; controlling blood pressure; and modulating the regional flow of blood to tissues. In performing these roles, eicosanoids most often act as autocrine signaling agents to impact their cells of origin or as paracrine signaling agents to impact cells in the proximity of their cells of origin. Eicosanoids may also act as endocrine agents to control the function of distant cells.

Leukotrienes are a family of eicosanoid inflammatory mediators produced in leukocytes by the oxidation of arachidonic acid (AA) and the essential fatty acid eicosapentaenoic acid (EPA) by the enzyme arachidonate 5-lipoxygenase.

Lipoxygenases are a family of (non-heme) iron-containing enzymes most of which catalyze the dioxygenation of polyunsaturated fatty acids in lipids containing a cis,cis-1,4- pentadiene into cell signaling agents that serve diverse roles as autocrine signals that regulate the function of their parent cells, paracrine signals that regulate the function of nearby cells, and endocrine signals that regulate the function of distant cells.
An antileukotriene, also known as leukotriene modifier and leukotriene receptor antagonist, is a medication which functions as a leukotriene-related enzyme inhibitor or leukotriene receptor antagonist and consequently opposes the function of these inflammatory mediators; leukotrienes are produced by the immune system and serve to promote bronchoconstriction, inflammation, microvascular permeability, and mucus secretion in asthma and COPD. Leukotriene receptor antagonists are sometimes colloquially referred to as leukasts.

Leukotriene C4 (LTC4) is a leukotriene. LTC4 has been extensively studied in the context of allergy and asthma. In cells of myeloid origin such as mast cells, its biosynthesis is orchestrated by translocation to the nuclear envelope along with co-localization of cytosolic phospholipase A2 (cPLA2), Arachidonate 5-lipoxygenase (5-LO), 5-lipoxygenase-activating protein (FLAP) and LTC4 synthase (LTC4S), which couples glutathione to an LTA4 intermediate. The MRP1 transporter then secretes cytosolic LTC4 and cell surface proteases further metabolize it by sequential cleavage of the γ-glutamyl and glycine residues off its glutathione segment, generating the more stable products LTD4 and LTE4. All three leukotrienes then bind at different affinities to two G-protein coupled receptors: CYSLTR1 and CYSLTR2, triggering pulmonary vasoconstriction and bronchoconstriction.
Arachidonate 5-lipoxygenase, also known as ALOX5, 5-lipoxygenase, 5-LOX, or 5-LO, is a non-heme iron-containing enzyme that in humans is encoded by the ALOX5 gene. Arachidonate 5-lipoxygenase is a member of the lipoxygenase family of enzymes. It transforms essential fatty acids (EFA) substrates into leukotrienes as well as a wide range of other biologically active products. ALOX5 is a current target for pharmaceutical intervention in a number of diseases.

Sterol regulatory element-binding protein cleavage-activating protein, also known as SREBP cleavage-activating protein or SCAP is a protein that in humans is encoded by the SCAP gene.

ALOX15 is, like other lipoxygenases, a seminal enzyme in the metabolism of polyunsaturated fatty acids to a wide range of physiologically and pathologically important products. ▼ Gene Function

ALOX12, also known as arachidonate 12-lipoxygenase, 12-lipoxygenase, 12S-Lipoxygenase, 12-LOX, and 12S-LOX is a lipoxygenase-type enzyme that in humans is encoded by the ALOX12 gene which is located along with other lipoyxgenases on chromosome 17p13.3. ALOX12 is 75 kilodalton protein composed of 663 amino acids.

G-protein coupled receptor 31 also known as 12-(S)-HETE receptor is a protein that in humans is encoded by the GPR31 gene. The human gene is located on chromosome 6q27 and encodes a G-protein coupled receptor protein composed of 319 amino acids.

Cysteinyl leukotriene receptor 1, also termed CYSLTR1, is a receptor for cysteinyl leukotrienes (LT). CYSLTR1, by binding these cysteinyl LTs contributes to mediating various allergic and hypersensitivity reactions in humans as well as models of the reactions in other animals.

Leukotriene B4 receptor 2, also known as BLT2, BLT2 receptor, and BLTR2, is an Integral membrane protein that is encoded by the LTB4R2 gene in humans and the Ltbr2 gene in mice.

Cysteinyl leukotriene receptor 2, also termed CYSLTR2, is a receptor for cysteinyl leukotrienes (LT). CYSLTR2, by binding these cysteinyl LTs contributes to mediating various allergic and hypersensitivity reactions in humans. However, the first discovered receptor for these CsLTs, cysteinyl leukotriene receptor 1 (CysLTR1), appears to play the major role in mediating these reactions.

Oxoeicosanoid receptor 1 (OXER1) also known as G-protein coupled receptor 170 (GPR170) is a protein that in humans is encoded by the OXER1 gene located on human chromosome 2p21; it is the principal receptor for the 5-Hydroxyicosatetraenoic acid family of carboxy fatty acid metabolites derived from arachidonic acid. The receptor has also been termed hGPCR48, HGPCR48, and R527 but OXER1 is now its preferred designation. OXER1 is a G protein-coupled receptor (GPCR) that is structurally related to the hydroxy-carboxylic acid (HCA) family of G protein-coupled receptors whose three members are HCA1 (GPR81), HCA2, and HCA3 ; OXER1 has 30.3%, 30.7%, and 30.7% amino acid sequence identity with these GPCRs, respectively. It is also related to the recently defined receptor, GPR31, for the hydroxyl-carboxy fatty acid 12-HETE.

5-Hydroxyeicosatetraenoic acid (5-HETE, 5(S)-HETE, or 5S-HETE) is an eicosanoid, i.e. a metabolite of arachidonic acid. It is produced by diverse cell types in humans and other animal species. These cells may then metabolize the formed 5(S)-HETE to 5-oxo-eicosatetraenoic acid (5-oxo-ETE), 5(S),15(S)-dihydroxyeicosatetraenoic acid (5(S),15(S)-diHETE), or 5-oxo-15-hydroxyeicosatetraenoic acid (5-oxo-15(S)-HETE).

Arachidonate 15-lipoxygenase type II is an enzyme that in humans is encoded by the ALOX15B gene. ALOX15B, also known as 15-lipoxygenase-2, is distinguished from its related oxygenase, ALOX15 or 15-lipoxygenase-1.

Leukotriene-B(4) omega-hydroxylase 1 is an enzyme protein involved in the metabolism of various endogenous substrates and xenobiotics. The most notable substrate of the enzyme is leukotriene B4, a potent mediator of inflammation. The CYP4F2 gene encodes the enzyme in humans.
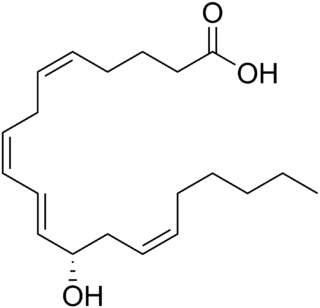
12-Hydroxyeicosatetraenoic acid (12-HETE) is a derivative of the 20 carbon polyunsaturated fatty acid, arachidonic acid, containing a hydroxyl residue at carbon 12 and a 5Z,8Z,10E,14Z Cis–trans isomerism configuration (Z=cis, E=trans) in its four double bonds. It was first found as a product of arachidonic acid metabolism made by human and bovine platelets through their 12S-lipoxygenase (i.e. ALOX12) enzyme(s). However, the term 12-HETE is ambiguous in that it has been used to indicate not only the initially detected "S" stereoisomer, 12S-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid (12(S)-HETE or 12S-HETE), made by platelets, but also the later detected "R" stereoisomer, 12(R)-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid (also termed 12(R)-HETE or 12R-HETE) made by other tissues through their 12R-lipoxygenase enzyme, ALOX12B. The two isomers, either directly or after being further metabolized, have been suggested to be involved in a variety of human physiological and pathological reactions. Unlike hormones which are secreted by cells, travel in the circulation to alter the behavior of distant cells, and thereby act as Endocrine signalling agents, these arachidonic acid metabolites act locally as Autocrine signalling and/or Paracrine signaling agents to regulate the behavior of their cells of origin or of nearby cells, respectively. In these roles, they may amplify or dampen, expand or contract cellular and tissue responses to disturbances.

Soluble epoxide hydrolase (sEH) is a bifunctional enzyme that in humans is encoded by the EPHX2 gene. sEH is a member of the epoxide hydrolase family. This enzyme, found in both the cytosol and peroxisomes, binds to specific epoxides and converts them to the corresponding diols. A different region of this protein also has lipid-phosphate phosphatase activity. Mutations in the EPHX2 gene have been associated with familial hypercholesterolemia.
13-Hydroxyoctadecadienoic acid (13-HODE) is the commonly used term for 13(S)-hydroxy-9Z,11E-octadecadienoic acid (13(S)-HODE). The production of 13(S)-HODE is often accompanied by the production of its stereoisomer, 13(R)-hydroxy-9Z,11E-octadecadienoic acid (13(R)-HODE). The adjacent figure gives the structure for the (S) stereoisomer of 13-HODE. Two other naturally occurring 13-HODEs that may accompany the production of 13(S)-HODE are its cis-trans (i.e., 9E,11E) isomers viz., 13(S)-hydroxy-9E,11E-octadecadienoic acid (13(S)-EE-HODE) and 13(R)-hydroxy-9E,11E-octadecadienoic acid (13(R)-EE-HODE). Studies credit 13(S)-HODE with a range of clinically relevant bioactivities; recent studies have assigned activities to 13(R)-HODE that differ from those of 13(S)-HODE; and other studies have proposed that one or more of these HODEs mediate physiological and pathological responses, are markers of various human diseases, and/or contribute to the progression of certain diseases in humans. Since, however, many studies on the identification, quantification, and actions of 13(S)-HODE in cells and tissues have employed methods that did not distinguish between these isomers, 13-HODE is used here when the actual isomer studied is unclear.




















